A comprehensive review of FITC/fluorescein and its applications in biomedical research. A summary of anti-FITC antibodies cited among the over 60,000 formal publications in Labome's Validated Antibody Database is also presented.
Fluorescein is a fluorescent tracer with wide applications in laboratory research (discussed below) and medical practice (such as fluorescein angiography [3] and fluorescein-guided surgery [4] ). In water, its maximum absorption/emission is 494/518 nm, corresponding to the color blue and green. The blue color spectrum is 450-495 nm, and the green spectrum is 495-570 nm. Fluorescein isothiocyanate (FITC) is the derivative of fluorescein with an isothiocyanate reactive group, rendering it reactive towards amine and sulfhydryl groups commonly found in biomolecules.
| Wavelength | Color | |
|---|---|---|
| maximum excitation | 494 | blue |
| maximum emision | 518 | green |
Labome has surveyed formal publications using FITC/fluorescein or FITC-conjugated reagents. The most common fluorescein applications are to conjugate with secondary antibodies and to detect apoptotic cells. Besides, many other moieties can be conjugated with FITC, and the conjugated complexes have been used in a variety of experimental procedures.
Fluorescein is one of the commonly used fluorescent labels for detection in flow cytometry and immunofluorescence (including both immunocytochemistry and immunohistochemistry experiments), through its conjugation with various secondary antibodies. A similar survey of secondary antibodies with a brief discussion of FITC can be found here.
FITC-conjugated secondary antibodies from Thermo Fisher, including brands Applied Biosystems, Life Technologies, Invitrogen, Molecular Probes, Biosource International, Zymed Laboratories, were used in flow cytometry or immunocytochemistry to investigate GDAP1 expression [5], the role of HLA-C in coronavirus HKU1 infection [6], the role of CD63 in renal and lysosomal functions [7], the Leber congenital amaurosis animal models [8], and the role of Smad4 in follicle stem cell maintenance [9].
AbD Serotec FITC-conjugated goat anti-human IgG was used to perform immunohistochemistry to examine synaptic plasticity [10].
FITC-conjugated anti-mouse or anti-rabbit secondary antibodies from BD Biosciences (BD Pharmingen) were used in flow cytometry or immunocytochemistry to examine the IL-17 immunodeficiency [11], the role of chemokines in cell homing [12], the role of CD63 in renal and lysosomal functions [7], the effect of autophagy on apoptosis [13], and LRRK2 subcellular localization [14].
Jackson Immunoresearch Laboratories is a reagent supplier focused on secondary antibodies. Its FITC-conjugated secondary antibodies were used in immunohistochemistry and immunocytochemistry to explore the role of Drosophila CREB binding protein in chromosomal function [15], the effect of HMGN1 on ATM activation [16], the Hif-alpha/Notch interaction [17], X chromosome inactivation [18], Ryk-ICD pathway in mutant polyglutamine neurons [19], and the role of RSFI in DSB repair [20], the usage of Poly-Ribo-Seq in translating the small open reading frames [21], the role of radial glial progenitors in stabilizing nascent brain vascular network [22], the mechanism of the glial responsiveness to axonal injury in Drosophila [23] and the role of human lariat RNA debranching enzyme 1 protein in the nucleus and cytoplasm [24].
MilliporeSigma FITC-conjugated anti-rabbit or anti-mouse secondary antibodies were used in immunostaining and flow cytometry to research matrix metalloproteinase-7 function [25], LRRK2 subcellular localization [14], the function of herpes simplex virus 1 ICP0 [26], the role of chemokines in cell homing [12], and the role of sFLT-1 in the maintenance of the avascular photoreceptor layer in mouse models [27].
Rhodes JDP et al used FITC-conjugated rabbit anti–sheep from Vector Laboratories (FI-6000) at 1:100 to detect sheep anti-digoxigenin from Roche (11207741910) in RASER (Resolution After Single-strand Exonuclease Resection)-FISH experiments [28].
One of the most popular applications of fluorescein is in apoptosis detection. Two main approaches are the direct labeling of the DNA fragments with fluorescein-tagged nucleotides ( TUNEL assay) and conjugation of the annexin with fluorescein ( annexin affinity assay). An additional method is to detect caspase activity through fluorescein-labeled pan-caspase inhibitor Z-VAD-FMK, as in CaspGLOW Fluorescein Active Caspase Staining Kit from Biovision [29].
ApopTag fluorescein in situ apoptosis detection kit (link) from MilliporeSigma (Millipore, Chemicon) is the most popular TUNEL assay system. It has been used in immunomicroscopy and flow cytometry to study the role for Drosophila Sir2 in cell survival and death [30], the effect of fidarestat [31], cardiomyocyte cytochrome P450 [32], DNA damages in A-T-like disease and Nijmegen breakage syndrome [33], and interleukin-1 receptor [34]. Rusty Kelley et al used Promega fluorescein-12-dUTP in TUNEL assay to study the function of Bmper in 2009 [35].
Annexin V can bind to phosphatidylserine, which, during cell death or apoptosis, is present on the outer leaflet of the cell membrane. Fluorescein-labeled annexin thus can be used to detect and quantitate the apoptotic/dead cells under microscopes or with flow cytometry. EBiosciences (Bender MedSystem) annexin-V FITC apoptosis detection kit was used to investigate the control of murine blood glucose [36], and the origin of human prostate cancer [37]. Annexin V conjugated with fluorescein from BD Biosciences (Pharmingen, Transduction Laboratories) was used in flow cytometry and immunomicroscopy to examine the effect of JAK2 mutations on hematopoietic stem cells [38]. MilliporeSigma / Roche Applied Science in situ cell death detection kit was used to examine the role of p53 in the differentiation of human embryonic stem cells [39].
Promega FITC-VAD-FMK (a pan-caspase inhibitor) was used for apoptosis assay to investigate microRNA155 [40] and ephrinB3 [41].
In addition to the widely used TUNEL assay employing fluorescein, fluorescein-tag nucleotides can also be used in cell proliferation assay ( BrdU-conjugated) in flow cytometry, or used as in situ hybridization probes or as a delivery check for RNA [42]. MilliporeSigma / Roche digoxigenin/FITC-labeled UTP was used in RNA synthesis to examine the molecular mechanism of bilaterian head development [43].
RA Heimeier, VS Hsia, and YB Shi in 2008 used Thermo Fisher 6-carboxyfluorescein-labeled TaqMan probes for quantitative PCR to study the roles for BRG1-associated factor 57 and BRG1-containing chromatin remodeling complexes in gene activation [44].
As with secondary antibodies, primary antibodies can be labeled with fluorescein in flow cytometry and immunofluorescence experiments, which can be detected directly and can be used for a downstream application such as cell separation. H Kim et al labelled cells with h FITCconjugated anti-BrdU antibody ( 11-5071-42) from Invitrogen to analyze the cell cycle profile [45]. Chopra S et al stained mouse bone marrow–derived dendritic cells with 1.25 μg/ml FITC-conjugated MHC-II antibody from Tonbo during flow cytometry [46]. Jordão MJC et al stained mouse brain and spinal cord sections with 3 ug/ml FITC-labeled mouse anti-SPARC antibody from R&D Systems [47]. Miltenyi anti-c-myc-FITC was used in yeast display selection to examine the structure of XWnt8-Frizzled-8 cysteine-rich domain complex [48]. Jackson ImmunoResearch FITC-conjugated mouse transferrin was used to perform transferrin binding assays to investigate virus-induced evolution of transferrin receptor [49]. Polysciences anti-FITC BioMag particles were used to perform the negative selection of T cells labeled with FITC-conjugated antibodies against CD8 or CD4, MHC class II, CD11b, B220, and CD25 [50].
FITC can be conjugated with anti-Tag antibodies such as c-Myc, His, and FLAG, and with other affinity reagents to stain different target moieties.
SJ Fleishman et al used Miltenyi Biotec FITC-conjugated c-myc antibody in flow cytometry in evaluating a computational method for influenza therapy in 2011 [51], and W Fang et al used Anaspec FITC-labeled His-tag antibody in immunocytochemistry to develop mosquito-killing transgenic fungi [52].
Avidin-biotin affinity system has been employed in a myriad of experiments. Both biotin and avidin/streptavidin can be linked with fluorescein to enable high fidelity detection. MilliporeSigma-Aldrich avidin-FITC conjugate was used to perform immunofluorescence to examine the role of mast cells in dengue virus-induced vascular leakage [53]. Vector Labs avidin-FITC was used in immunohistochemistry to explore genomic features of germinal cell lineages in Schistosomes [54]. Zymed FITC-conjugated avidin was used to perform immunohistochemistry to investigate mast cells with A20 deficiency [55].
Phalloidin can bind to F-actin with high specificity. MilliporeSigma-Aldrich FITC-phalloidin conjugate was used for confocal microscopy to study the bidirectional differentiation potentials of vascular calcifying progenitor cells [56].
Lectins are a group of the proteins that can bind to carbohydrate moieties with high affinity. Silvestre-Roig C et al stained plasma membranes with FITC-conjugated Triticum vulgaris lectin [57]. Fluoresceinated isolectin B4 is commonly used to stain vascular endothelial cells [58].
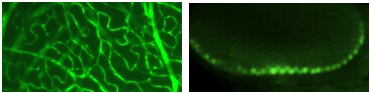
Fluorescein can also be conjugated to large molecules to examine the integrity of cell membranes and tissue junctions. One typical molecule is dextran. The movement of FITC-dextran serves as a measure of the blood-brain barrier permeability [59], colonic epithelial barrier permeability [60, 61], or macropinocytosis [62]. Other tracers have also been used for studying the integrity of the blood-brain barrier, such as Alexa Fluor 555 cadaverine [63]. MilliporeSigma fluorescein isothiocyanate-dextran was used to examine intestinal permeability [64]. Molecular Probes FITC-dextran dye was used to investigate the translation of a subset of mRNAs encoding secretory proteins potentiated by RanBP2/Nup358 [65].
| Supplier | Num |
|---|---|
| Thermo Fisher | 17 |
| Abcam | 3 |
| MilliporeSigma / Roche Applied Science | 3 |
| Vector Laboratories | 1 |
Both in situ hybridization and immunohistochemical signals can be enhanced through the fluorescein tyramide system. Perkin-Elmer TSA Fluorescein Tyramide Reagent Pack was used to investigate Hippo signaling [66]. Thermo Fisher FITC-DQ-collagen I was used to perform an activity assay to investigate tumor microenvironments [67]. IA Klein et al studied the partitioning behavior of fluorescein in an in vitro droplet assay to investigate the retention of small molecule pharmaceuticals in nuclear condensates [68].
Ma L et al took advantage of the existence of a chimeric antigen receptor recognizing fluorescein isothiocyanate to test a vaccine strategy to improve the efficacy of CAR–T cells [69].
| Method | Supplier | Catalog number | Sample reference |
|---|---|---|---|
| IC | Invitrogen | A-11090 | [70] |
| IHC | Invitrogen | A-11096 | [71, 72] |
| IHC | Abcam | ab6655 | [73] |
| IHC | Invitrogen | A-11095 | [71] |
| IHC | Invitrogen | ANZ0109 | [74] |
| IHC | MilliporeSigma | 11426346910 | [75] |
| IHC | Vector Laboratories | SP-0601 | [76] |
| IHC-F | Invitrogen | 71-1900 | [77] |
| IHC-P | Invitrogen | 71-1900 | [78] |
| IHC-P | Invitrogen | A-11090 | [79] |
| IHC-P | Invitrogen | A-889 | [80] |
| WB | Abcam | ab6213 | [81] |
Large molecules conjugated with fluorescein can be used to trace microvessels. Nortley R et al measured the diameters of brain microvessels with FITC-albumin [82]. Mauffrey P et al studied the integrity of the blood–brain barrier in the subventricular zone of Hi-MYC cancer mice with FITC-albumin [83].
FITC can be directly used to paint and label small animals and microorganisms [27] or cerebral metastases during neurosurgeries [84].
Derivatives of fluorescein have exciting applications. Fluorescein diacetate, in conjunction with propidium iodide, is often used to stain live/dead cells [85]. Fluoro-jade dyes (Fluoro-Jade, Fluoro-Jade B, and Fluoro-Jade C) are used to stain dead or dying neurons [86]. The cell-permeant 2',7'-dichlorodihydrofluorescein diacetate (DCF), a reduced form of fluorescein, is used as an indicator for reactive oxygen species inside cells, for example, to detect the generation of reactive oxygen intermediates in bone marrow mouse neutrophils undergoing netosis [57], peritoneal macrophages [87]. 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) is a pH indicator for acidic pH range. CFSE binds to the intracellular molecules covalently once inside a cell, and thus enables cell tracking and cell proliferation/death measurement [88-90]. Perkin Elmer Fluorescein TSA Plus Evaluation Kits were used to perform DNA labeling to examine the interaction between NK4 and Tbx1/10 in regulating cardiac versus pharyngeal muscle fate in the ascidian second heart field [91].
MilliporeSigma-Aldrich fluorescein cadaverine [92] and inulin [93] have also been used in various applications.
FITC signals can be detected either directly through fluorescence microscopes or an anti-FITC antibody. The utilization of anti-FITC antibodies can amplify the FITC signals and/or eliminate the quenching of FITC fluorescence. For example, Ma L et al detected the in vitro surface labeling of the amphiphile-FITC conjugate in cells through flow cytometry with an anti-FITC monoclonal antibody (clone: 1F8-1E4) from Jackson Immunoresearch [69]. Labome has surveyed over 60,000 formal publications to develop Validated Antibody Database, as of Dec 28, 2018. Table 2 lists the suppliers of anti-FITC antibodies among the articles surveyed, and Table 3 displays the catalog numbers for specific methods.
- Li B, Zhang H, Shi Y, Min Y, Lin S, Wu K, et al. Overexpression of nuclear transport factor 2 may protect against diabetic retinopathy. Mol Vis. 2009;15:861-9 pubmed
- Pedrola L, Espert A, Valdés Sánchez T, Sanchez Piris M, Sirkowski E, Scherer S, et al. Cell expression of GDAP1 in the nervous system and pathogenesis of Charcot-Marie-Tooth type 4A disease. J Cell Mol Med. 2008;12:679-89 pubmed
- Drel V, Pacher P, Ali T, Shin J, Julius U, El Remessy A, et al. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21:667-76 pubmed
- Ikeda J, Wada N, Nojima S, Tahara S, Tsuruta Y, Oya K, et al. ID1 upregulation and FoxO3a downregulation by Epstein-Barr virus-encoded LMP1 in Hodgkin's lymphoma. Mol Clin Oncol. 2016;5:562-566 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Antibody Applications
- Antibody Companies
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- Flow Cytometry - A Survey and the Basics
- Fluorescent Sensors for Reactive Oxygen Species and Enzymes
- GFP Antibody
- Live Cell Imaging
- Secondary Antibodies Companies
- Secondary Antibodies
- The Glymphatic and Meningeal Lymphatic System
