Stem cells (SC) are defined by their remarkable ability to both self-renew in an undifferentiated state and have the potential to develop into mature somatic cell types both in vivo and in vitro. Four main types of SCs differ in the kind of mature cells they can generate: Embryonic SCs (ESCs) are pluripotent and can generate all somatic cell types [3]. Induced pluripotent stem (iPS) cells are somatic cells that have been reprogrammed to a pluripotent state and are thought to share crucial ‘stemness’ factors with ESCs [4]. Adult SCs (ASCs) such as hematopoietic stem cells (HSCs) are, however, restricted in their differentiation potential usually to their tissue of origin [5]. Finally, some of the cells found in cancers display properties and express similar markers to stem cells; these cells (Cancer stem cells (CSCs)) may drive tumorigenesis. Table 1 lists some of mouse proteins involved in stem cells.
| Sym | Protein | Top three suppliers |
|---|---|---|
| Arhgef2 | rho/rac guanine nucleotide exchange factor (GEF) 2 | Cell Signaling Technology 4076 (9), Abcam ab90783 (4) |
| Bmi1 | Bmi1 polycomb ring finger oncogene | Cell Signaling Technology 5856 (8) |
| Cdkn2a | cyclin dependent kinase inhibitor 2A | Abcam ab54210 (19), Santa Cruz Biotechnology sc-377412 (5), Invitrogen MA5-17142 (4) |
| Fgf13 | fibroblast growth factor 13 | Neuromab 75-246 (3) |
| Fgfr1 | fibroblast growth factor receptor 1 | Cell Signaling Technology 9740 (26), Novus Biologicals NB600-1287 (3), Santa Cruz Biotechnology sc-57132 (3) |
| Fgfr2 | fibroblast growth factor receptor 2 | Santa Cruz Biotechnology sc-6930 (4), Abcam ab109372 (1) |
| Fubp1 | far upstream element (FUSE) binding protein 1 | Santa Cruz Biotechnology sc-374342 (2) |
| Fzd7 | frizzled class receptor 7 | Santa Cruz Biotechnology sc-398082 (1) |
| Hoxb4 | homeobox B4 | Developmental Studies Hybridoma Bank I12 anti-Hoxb4 (1) |
| Kit | KIT proto-oncogene receptor tyrosine kinase | Invitrogen 14-1171-81 (49), BioLegend 105802 (48), BD Biosciences 553352 (34) |
| Lef1 | lymphoid enhancer binding factor 1 | Cell Signaling Technology 2230 (41), Abcam ab137872 (8) |
| Lrp6 | low density lipoprotein receptor-related protein 6 | Cell Signaling Technology 3395 (12), Santa Cruz Biotechnology sc-25317 (8), Abcam ab134146 (6) |
| Notch1 | notch 1 | Cell Signaling Technology 4147 (73), Abcam ab52627 (23), Invitrogen MA5-11961 (18) |
| Numb | NUMB endocytic adaptor protein | Cell Signaling Technology 2756 (10), Santa Cruz Biotechnology sc-136554 (2) |
| Rab10 | RAB10, member RAS oncogene family | Cell Signaling Technology 8127 (14), Enzo Life Sciences ALX-804-220-R100 (9), Abcam ab230261 (6) |
| Tgfb2 | transforming growth factor, beta 2 | Abcam ab190503 (1) |
| Vangl2 | VANGL planar cell polarity 2 | Santa Cruz Biotechnology sc-515187 (1) |
| Wnt3a | wingless-type MMTV integration site family, member 3A | Cell Signaling Technology 2721 (6), Abcam ab81614 (3), Santa Cruz Biotechnology sc-136163 (1) |
| Zfp36l2 | zinc finger protein 36, C3H type-like 2 | Santa Cruz Biotechnology sc-365908 (1) |
Stem cells have great potential for treating human diseases and as such a vast number of studies have been and are being focused on stem cell research. The mouse has been the leading model system to study stem cell biology, which makes them extremely useful in the advancement of our understanding of possible therapeutic uses of these cells. For example, hematopoiesis research utilizes mouse models extensively, such as epigenetic memory in immune responses [6].
After building on experience with teratocarcinoma cells lines, in 1981, two groups independently isolated and cultured the first mESCs [7, 8]. ESCs comprise the inner cell mass (ICM) of the embryo before they lose their pluripotency and become committed to particular cell fate. ESCs are now the most studied type of SCs, a great deal of which has been done in the mouse. Though the efficiency of ES derivation seems to be strain dependent, the mESCs are obtained by a relatively straight forward method: Mouse blastocysts (e3.5) are flushed from the uterine horns of the female mouse, and the zona pellucida is dissolved in 0.5 mL acid Tyrode’s solution. The zona pellucida-free blastocysts are then cultured for several days on mitomycin C-inactivated (or irradiated) confluent mouse embryonic fibroblast (MEF) feeder cells that have been seeded several hours (or overnight) before use. The blastocysts proceed to attach, and both trophoblast and ICM cells divide after attachment. Once the ICM outgrowths are around three to four times the size of the blastocyst, the outgrowths are picked, dispersed by careful enzymatic treatment with trypsin/EDTA, and re-plated (Figure 1). Under appropriate conditions, a percentage of the isolated outgrowths will continue to divide and maintain an undifferentiated ES cell morphology. The factors provided by the MEFs include, but are not restricted to, leukemia inhibitory factor (LIF) and bone morphogenic protein 4 (BMP-4). Most cell lines also require these factors, which help retain a state of undifferentiated self-renewal, to be supplemented in the culture medium in addition to that secreted from the MEFs. Although culture on MEF feeder cells is the standard and more traditional protocol, there are now different protocols known to allow the derivation and maintenance of undifferentiated mESCs successfully. Some mESC lines tolerate growth without MEF feeder cells though many will differentiate rapidly despite the presence of LIF in the culture medium. The MEF-free method replaces the feeder layer by coating dishes with 0.1% gelatin 30 minutes before plating the mESCs [9, 10]. Additional methods include the use of conditioned media [11] and the application of cytokines and different inhibitors [9, 12, 13]. In addition to exogenous factors mentioned above, there is a particular transcriptional network that sustains mESC self-renewal. This network includes Oct4, Nanog, and Sox2 and each factor stimulates the expression of itself and the others, constituting a positive feedback loop [14]. Co-culture of embryonic stem cells with their extraembryonic counterparts, trophoblast stem cells, resembles mammalian embryogenesis more than the culture of embryonic stem cells alone. Thus the co-culture presents a new model for developmental studies [15]. For an example, He M et al derived mESCs from blastocysts cultured on irradiated MEF feeder cells (Millipore Sigma) in ESGRO-2i medium (Millipore Sigma SF016-100) and maintained them on feeder cells in KNOCK-OUT medium [16].
Once the mESCs have been isolated, and a cell line has been established, the cells need to be characterized. There are several ways to do this including karyotyping, embryoid bodies and mouse chimeras. The cells need to be karyotyped to ensure that they are euploid and their pluripotency can be determined both in vitro and in vivo using different assays. ES cells can form embryo-like aggregates in suspension culture that consist of derivatives of the three embryonic germ layers (endoderm, mesoderm, and ectoderm) and are known as embryoid bodies [17]. For in vivo testing, mESCs are injected into immune-compromised mice and can form teratomas that contain tissues that originate from the three germ layers. The generation of chimeric mice by injection of mESCs into a normal blastocyst with derivatives of the injected cells appearing in all organs including the germline further illustrates their potential to contribute to all tissues of the adult organism [18]. Several commercial organizations can perform these tests for the research community.
One of the most common applications of mouse ES cells is to create mouse models by genetic modifications via the introduction of foreign DNAs into the ES cells. There are several ways to carry out gene delivery to these cells including both viral and non-viral routes. In viral delivery, the backbone of a viral genome (e.g., adenovirus and lentivirus) is used, which has an insertion of the gene(s) of interest to be expressed in the stem cell. The non-viral route is more commonly used and includes electroporation, lipofection, and nucleofection-based techniques. The modified ES cells can then be used to create chimeras as detailed above. Another appliction is to generate organoids whose three-dimensional structures mimic the physiological tissues or organs to certain degrees [19].
A new protocol for developing 3D trophoblast organoids from naïve human pluripotent stem cells (hPSCs) has recently been published [20]. The generated organoids contained distinct cytotrophoblast, syncytiotrophoblast and extravillous trophoblast cell layers, which structurally resembled human embryo. The authors described the analysis of organoids by immunofluorescence, flow cytometry, mRNA and microRNA expression profiling, and placental hormone secretion. In addition, the generated trophoblast organoids differentiated into extravillous trophoblast organoids, which showed solid invasive capacity when co-incubated with human endometrial cells.
A single-cell genomic analysis of gastruloids developed from pluripotent stem cells has recently been reported [21]. The study monitored symmetry breaking during gastruloid development and showed spatial variability in pluripotency. The cells in the center of the gastruloid returned to pluripotency, while the peripheral cells differentiated into the primitive streak-like cells. These cell populations broke radial symmetry and induced axial elongation. In addition, using a dual Wnt modulation, the authors optimized the generation of anterior structures in the existing gastruloid model.
There are now many mES cell lines available derived from various mouse strains that have been genetically manipulated to be used in mouse modeling of different diseases, for example, V6.5 [22], though it is evident that the genetic background of the ES cells is vital for specific disease models. Discrepancies due to different genetic backgrounds may arise when comparing phenotypes of genes targeted in ES cell lines. It is therefore essential to choose carefully which lines will be used in specific areas of research [23]. In terms of disease-associated research the different problems associated with the background can be a motivator to use other types of stem cells such as iPSCs (see below).
The Jackson Laboratory has several commercially available mES cell lines available to the research community. The Laboratory also distributes knockout ES cell lines generated by Deltagen. Among others, Invitrogen also offers mES cell lines that constitutively express GFP under the influence of a specific promoter. A GFP-expressing ES cell line could be invaluable in transplantations and tissue replacement studies. There have been recent advances in the production of mES lines that are engineered for the forced induction of transcription factors (TFs). The TFs that were manipulated in these lines were chosen due to their involvement in critical functions of mES cells and their differentiation such as Sox2, Sox9, and Nanog. TF-manipulable ES cell lines could be used to study the complex mechanisms of ES cell differentiation toward specific lineages [24]. The National Institute of Aging provides an extensive list of the currently available transgenic mES cell lines that were generated in this study.
There are some limitations in the generation and use of mESCs. Unfortunately, not all mouse strains are permissive for ES cell derivation: Only a limited number of mouse strains, such as 129, C57BL/6, and BALB/C allow for mESC isolation. This makes it harder to investigate gene function and pathogenesis in other strains. Therefore applicability of ES cell principles across species needs to be taken into consideration.
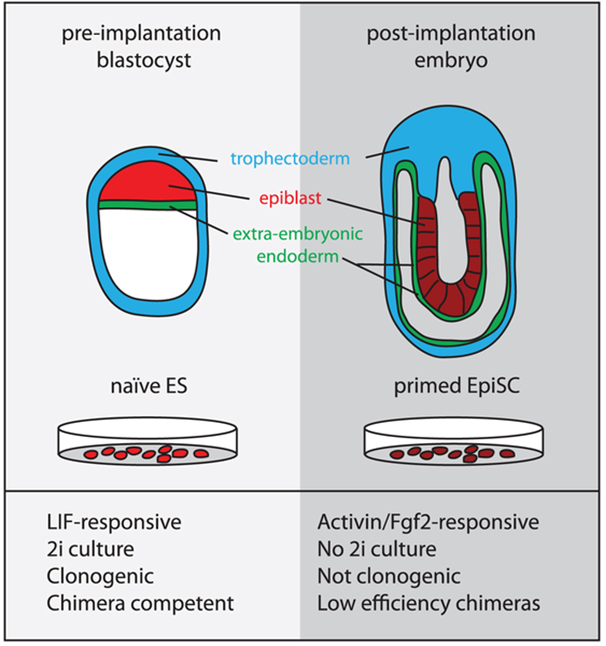
An additional type of pluripotent cell has been isolated from the mouse epiblast which is the single layer of epithelial cells that originates from the ICM after implantation of the embryo (Figure 2). These cells (mEpiSCs) are distinct from mESCs in terms of molecular and epigenetic signatures and are termed primed and naïve SCs respectively [25] ; although mEpiSCs are pluripotent they seem to have a more limited developmental potential in comparison to mESCs. This recent discovery provides a new perspective on the use of these cells in research as mEpiSCs are much more similar to human ES cells than mES cells. They share similar characteristics with hESCs such as the flattened two-dimensional (2D) colony morphology and are maintained under similar growth factor conditions. The close match between mEpiSCs and human ES cells could mean a functional similarity between these cells. mEpiSCs, like mESCs, express Oct4, Sox2, and Nanog and are capable of generating derivatives of all three germ layers during both in vitro differentiation and in vivo teratoma formation. They do not, however, contribute to chimera formation when injected into recipient blastocysts and whereas mESCs require LIF and BMP4, mEpiSC and hESCs require a combination of bFGF, ActivinA, or TGFβ and also activation of the Wnt signaling pathway [26].
Cell fate has been manipulated experimentally for several years beginning with the frog more than 50 years ago. When a somatic cell nucleus is transferred to an oocyte with its nucleus, the somatic fate is removed giving the oocyte an embryonic or pluripotent state. This method of reversal from a differentiated state to a pluripotent state is known as somatic cell nuclear transfer (SCNT). For several years this was the primary technique for generating patient-specific cell lines for therapeutic applications. The pluripotent stem cells generated from SCNT would be isogenic and thus not susceptible to immune rejection after transplantation of the differentiated cells back to the same donor and was, therefore, an attractive technique. Several mouse models were generated as a proof of concept through the generation of human SCNT ESCs has been more challenging [27]. A more practical and successful approach using iPS cells may, however, be the key to overcoming problems with SCNT.

The reprogramming of murine somatic cells was first reported in 2006 by Takahashi and Yamanaka [28]. Using retroviral vectors to induce forced expression of four transcription factors (Oct4, Sox2, Klf4, and cMyc), they were able to reprogram MEFs to iPSCs. These four factors have now also been shown to induce pluripotency in human somatic cells. Mouse iPSCs are highly similar to mESCs including genetic and epigenetic signatures (e.g., methylation patterns of Oct4 and Nanog), pluripotency marker expression, teratoma formation, chimera contribution, and germline transmission. Also, they also rely on the same signals to maintain pluripotency, i.e. LIF. Thus iPSCs are incredibly similar to mESCs, and importantly human iPSCs have been successfully generated from healthy and diseased individuals providing a more practical approach than SCNT.
Since these first studies using MEFs, iPSCs have been generated from several other tissues including keratinocytes, pancreatic islet cells, B lymphocytes, bone marrow cells, liver cells, neural stem cells, and meningiocytes [29]. There are now several alternative ways for gene delivery that may be safer than retroviral vectors and prevent the introduction of transgenes into the genome. These include non-integrating adenoviruses or transient plasmid transfection and the piggyBac (PB) transposon gene delivery systems [30]. Also, there are also several advantages of using iPSCs over ESCs. Firstly iPSCs are generated from the autologous recipient and thus avoids the host immunity problems in terms of transplantations. Another significant benefit is that it circumvents the ethical concerns of ESC use.
Due to the ability to generate entirely iPS-derived viable progeny by tetraploid complementation these cells can be useful tools to develop transgenic mouse strains with specific gene defects homologous to those seen in human pathology. These disease-specific iPS cells can be further used to explore given disease mechanisms both in vitro and in vivo (Figure 3). For example, a mouse (New Zealand Black) model for human chronic lymphocytic leukemia (CLL) (CD5+ B-cell malignancy) which exhibits a defect in the miR-15a/16–1 gene could not be used when generated from ES cells due to the refractory nature of this strain but was able to be modeled with iPS cells from spleen stromal cells [31]. Another study took iPS cells derived from human skin fibroblasts that had a disrupted CCR5 locus which is known to be a co-receptor for HIV entry. The iPS cells were differentiated into CD34+ cells that formed all types of hematopoietic colonies and could then be transplanted into mouse recipients to study the role of CCR5 in HIV and AIDS [32]. A further study has shown that using a sickle cell anemia mouse model and autologous iPS cells with a corrected sickle hemoglobin allele, followed by differentiation into HSCs and transplantation into irradiated mice. The mice could be rescued from disease progression [33]. There have been several additional mouse models used in iPSC studies for the treatment of disease including type 1 and type 2 diabetes [34]. This work has tremendous potential in providing an approach to study human disease in animal models not least that they have been obtained from every human somatic tissue and also from a range of diseased tissues. Despite this, care must be taken in terms of the differences between miPSCs and hiPSCs and also several concerns have been highlighted about the safety of using iPSCs with respect to compromised genomic integrities. Thus before they can be used in the clinic, further insight is required into the exact mechanisms underlying the cells’ identity. One very recent study that aims to advance this area of research generated the ROSA26 iPSC mouse; a mouse model that allows for the replacement of the four reprogramming factors in the iPSCs with specific genes of interest [35]. It is clear that this area of iPSC research has advanced at unprecedented rates and that the use of patient-specific iPSCs will have a significant impact on the study of human diseases yet a deal of work remains to be done before use in a clinical setting.
Unfortunately many therapeutic approaches for cancer do not eliminate all tumor cells, and as a result the remaining cells often cause a recurrence or metastasis of the tumor. There have been several recent studies proposing the presence of cancer stem cells (CSCs) as an explanation of the origin of cancer. In some solid cancers, cells have been identified that have similar functional properties and markers to stem cells. These include continuous proliferation, self-renewal, and expression of essential stem cell genes. These cells are not named CSCs due to their origin but because of their similarities with stem cells. The current view on CSCs is that adult stem cells, progenitor cells, or differentiated cells can acquire the genetic and epigenetic alterations to become CSCs involved in oncogenesis.
In mouse, CSCs have been suggested for a while in myeloma and acute myeloid leukemia, and for the latter, CSCs were identified in human in 1997 for the first time after transplantation of a CD34+CD38– cell subpopulation by into immunocompromised (NOD/SCID) mice [36]. Following this, CSCs have been identified in several human cancers including breast [37], prostate [38], intestinal [39] and brain [40] cancers among a handful of others. Identification of these cells, however, could be tricky, despite markers such as CD44 and CD133 being detected in a broad range of tumors, there seem to be several tissue-specific markers expressed, for example, the cell adhesion receptor, Integrin α2β1, appears to be specific for prostate CSCs [41]. To this end, mouse models of cancer have the potential to provide much-needed information about cells of origin of particular cancers.
The CSC population in breast cancer tumors has been studied in several mouse models [42, 43]. One example is the p53-null mouse mammary gland tumor model which is thought to closely resemble human breast cancer [44]. In this model, a subpopulation of tumor-inducing cells was identified and shown to express CD24 and CD29 (Lin-CD24HCD29H). In this study, the researchers used in vitro mammosphere assays and subsequent transplantation in vivo to identify these as CSCs. Transplantation of this sub-population into normal healthy mice generated heterogeneous tumors that resembled the primary tumor from which they were isolated.
Another example is the MMTV-Wnt1 mouse model where a Thy1+CD24+ CSC population was found to be highly enriched for CSCs [42]. In this isolated population, one in every 200 cells generated tumors upon mammary fat pad transplantation and had a similar phenotype to the primary tumor. Recently, a mouse model of triple-negative breast cancer revealed a connection between the loss of p53 and the expansion of the CSC pool [45].
In a recent mouse model of prostate cancer, it was shown that malignant neoplasms arise from the proximal region of the prostatic ducts which is an area enriched in stem cells [46]. The early cells of the neoplasms were shown to express the stem cell marker Sca1. It had been shown earlier by this group that in this model of prostate cancer where p53 and Rb were conditionally inactivated, that these genes play a role in prostate carcinogenesis. In another model where Pten was conditionally inactivated, it was shown that stem cell proliferation was directly associated with prostate tumor initiation and progression. When these two mouse models are compared, although they are both on the same transgenic mouse strain (PB-Cre4), they exhibit differences in the types of neoplasms generated indicating that the mechanisms of transformation of stem cells in the two models are quite distinct. This suggests that studying multiple transgenic mouse models of each cancer type is required.
The two most common brain tumors are gliomas and medulloblastomas, and there have been a variety of mouse models of these types of tumors generated to date including those with mutations in Pten, Ras, Wnt, and Patched (Ptch) among other genes [47]. One group, using the Patched mutant model of medulloblastoma showed that the tumors, in the mouse, are propagated by cells expressing the progenitor markers Math1 and CD15/SSEA-1 and not by CD133+ cells which had been previously implicated in human brain tumors as CSCs [48]. Thus there may be some discrepancy between mouse models and human disease which needs to be addressed.
Since the identification of possible cancer-inducing CSCs, several years ago, there has been extensive research to try and isolate and target these cells in a bid to advance the treatment of human cancers. It seems, however, that much more research is needed especially as it is apparent that there are many tissue-specific CSCs and with the lack of research into the CSC niche, much remains to be elucidated. Rapid advances are being made with mouse models of different cancers, and further generation and study of these models should provide more insight into the clinical relevance of CSCs in a broad range of human tumors.
Due to the differences between humans and animal models of disease development and the ethical issues surrounding non-human primates, there has been a growing demand for new animal models that allow for the study of human tissues and cells in vivo. This has led to the use of humanized mouse models for stem cell therapy which also permits testing of new human SC-based therapies for safety and treatment efficacy in mice with the related disease. Humanized mice are defined as immunodeficient mice engrafted with human CD34+ HSCs. The generation of SCID mice, which have a lack of T- or B-cells but still a high NK-cell activity, followed by the generation of mice that have a SCID mutation on the NOD background and additionally have a marked decrease in NK-cell activity has dramatically advanced this area of research. In addition to SCID-NOD mice, there are also newer improved versions including the NSG [49], NOG and RAG mice (reviewed in [50] ). For example, Calvanese V et al assessed the in vivo reconstitution ability of cultured hematopoietic stem or progenitor cells derived from human fetal liver or cord blood in NSG mice from Jackson Laboratories [49].
Humanized mice have been used to study several diseases including human immunodeficiency virus (HIV), arthritis, lung injury, and spinal cord injuries. One such example in an HIV prevention study used the NOD-SCID mice and carried out rectal exposure to HIV-1. A subset of mice was pre-treated with tenofovir, an antiretroviral gel — this reduced rectal transmission of HIV-1 from around 50% to 8% [51]. A recent study looking at rheumatoid arthritis (RA) also used humanized mice generated on the NOD-SCID background. The researchers induced arthritis in these mice via injection of Freund’s adjuvant into their joints. Before the initiation of arthritis one group of mice were treated with the TNF inhibitor, Etanercept, which is the most common therapy for RA patients. The Etanercept-treated mice exhibited less joint inflammation [52]. Another model generated using the NOD-SCID mice looked at spinal cord injury and transplantation of neuroepithelial-like stem cells generated from human iPS cells. The transplantation of these cells resulted in differentiation into neural lineages and promoted recovery of limb motor function [53]. A similar experiment using human adipose-derived SCs in transplantation to treat acute lung injury resulted in anti-inflammatory responses and attenuation of lung injury [54]. This is but a few examples of current humanized mice being used in disease-related research; there are several different models for a plethora of other diseases (Table 2).
| Phenotype | NSG | NOD-SCID | B6-RAG1 |
|---|---|---|---|
| B cells | Absent | Absent | Absent |
| T cells | Absent | Absent | Absent |
| Macrophages | Defective | Defective | Present |
| NK cells | Absent | Defective | Present |
| Irradiation tolerance | Low | Low | High |
| References | [55] | [56] | [57] |
It seems that current mouse models have come a long way since the human-mouse chimeras were first described. There are, however, still several limitations, including residual mouse immunity, which needs to be overcome. Despite this, humanized mice are currently and will continue to advance our knowledge of human disease response.
Mouse models are applied to study the applications of stem cells for the therapy of posttraumatic arthritis. The symptoms of osteoarthritis were significantly decreased due to the trophic effects of the engrafted cells in C57BL/6 mice with induced joint fracture injected with MSCs [58]. Inhibition of the development of destructive arthritis was observed in DBA1J mice immunized with collagen type II and treated with a combination of MSCs and IL-10-secreting regulatory T cells [59].
Transplantation of human placenta-derived MSCs has been shown to protect against nephritis and systemic inflammation in lupus nephritis-prone MRL/lpr mouse model [60]. Stem cells suppressed the production of anti-dsDNA antibodies and proteinuria and decreased the histological symptoms of nephritis. MSCs inhibited the expression of the pro-inflammatory genes, such as nuclear factor kappa B (NF-κB) and TNFα. Adipose-derived stem cells decreased the production of anti-dsDNA antibodies and IL-17 and the expression of mTOR and protein kinase B (Akt) in a mouse model of lupus [61].
Mouse models were used to evaluate applications of MSCs for the therapies of diabetes and atherosclerosis. Injection of MSCs into diabetic mouse models demonstrated correction of glycemic parameters, decreased pancreatic inflammation, increased regeneration of β cells. TGF-β engineered MSCs were shown to restore insulin production and suppress pancreatic inflammation in BALB/c mice with induced diabetes type 1 [62]. Also, since diabetes type 2 impairs vascularization and bone regeneration, diabetic mice (Lepr(db-/-)) with tibial defects were treated with mouse adipocyte-derived MSCs. The treatment significantly improved osteogenesis and angiogenesis in diabetic mice [63]. The effects of MSCs on the development of atherosclerosis were studied in low-density lipoprotein (LDL)-receptor knockout mice [64]. The study showed that the treatment with MSCs significantly reduced the formation of inflammatory lesions and lowered serum cholesterol.
Interestingly, extracellular vesicles isolated from human umbilical cord MSCs suppressed osteoarthritis development and osteophyte production and decreased the expression of ADAMTS5 and MMP13 in mouse knee joints in a mouse model of surgically induced osteoarthritis [65].
The effective prevention of the development of type 1 diabetes in a NOD mouse model by systemic MSC treatment has been reported [66]. MSCs suppressed infiltration of the islets by T cells and CXCL9-positive macrophages, and MSC treatment significantly improved the insulin content and β cell area [66]. Ex vivo-modulated HSCs showed strong migratory properties to sites of inflammation in a mouse model of type 1 diabetes and suppressed autoimmune diabetes in NOD mice [67]. In particular, HSCs decreased immune infiltration of pancreatic β cells and inhibited the functions of autoreactive T cells [67].
Spermatogonial stem cells (SSCs) are the stem cells of the male germ line. They support the continual production of sperm throughout the adult life of a male and are similar to ESCs in terms of their ability to self-renew and also to differentiate. They can regenerate spermatogenesis after various types of damage to the testis [68]. Two different approaches show the regenerative capabilities of SSCs. One route is via damage to spermatogenesis, for example, by the use of an alkylating agent, after which the SSCs will repopulate the testis and generate all the differentiated germ cells. The second approach is spermatogonial transplantation which can detect functional SSCs. The transplantation technique allows only those cells that continuously self-renew and produce the progenitor cells to regenerate spermatogenesis within the testis. With the use of the transgenic expression of different markers in donor cells such as GFP or LacZ, the resulting germ cell colonies generated by the SSCs can be readily detected. Markers of SSCs include GFRα, Nanos, E-Cad, and Plzf. Mouse SSCs, like mESCs, can be maintained in vitro virtually indefinitely and expand exponentially over time though they need to be exposed continuously to GDNF and fibroblast growth factor 2 (FGF2).
SSC transplantation has been shown to restore spermatogenesis in many species [69]. This could prove to be an invaluable tool particularly following oncological treatments that lead to infertility. Although there are already protocols in place for the cryopreservation of sperm from men, this is not always possible especially if the cancer is in a child. In this case, a biopsy must be taken. Promising studies in mice have shown that SSC transplantation can be used to treat infertility [70]. Recent developments have shown similar results in higher species including the monkey [71]. Using tissue from cancer patients, however, has its limitations due to the potential risk of contamination of isolated testicular cells with malignant cells. Recent work using mouse models has shown that it may be possible to eliminate contaminant cells from the SSC suspension using different markers for cancer cells [69]. Also, within the last few months, human-to–nude mouse xenotransplantation was used to assess SSC activity and malignant contamination in fractions obtained by FACS of contaminated human testis cell suspensions. This provided a method to isolate and enrich human SSCs, remove malignant contamination and succeed in repopulating the mouse testis [72]. This is a highly promising advancement in fertility research.
In addition, a novel therapeutic method for nonobstructive azoospermia has recently been suggested [73]. Mutation site in SSCs was repaired by using CRISPR-Cas9-mediated technique and engrafted into the testis. The treatment resulted in restoring spermatogenesis.
Microscopic embryonic-like stem cells (VSELs) were first described in adult mouse bone marrow [74] and subsequently in human tissue [75]. These are rare, tiny cells that express Sca-1 (mice) and CD133 (humans) but are negative for the hematopoietic markers Lin-1 and CD45. They express several markers that are associated with pluripotency such as Oct-4, Nanog, SSEA-1, Rex-1 and Rif-1 [76].
Mouse models of pancreatectomy have shown the involvement of VSELs in pancreatic regeneration [77]. For a recent overview and discussion of the therapeutic potential of VSELs see [78].
Mesenchymal stem cells (MSCs) are adult multipotent, non-hematopoietic stem cells. MSCs are an attractive source of stem cells for several reasons. They can be obtained from a range of mammalian sources including mice and humans; they are genetically stable; they can be readily expanded in cell culture; they can generate a variety of tissue types; and, they are free from the ethical issues associated with the use of ESCs. MSCs derived from different sources and expanded in cell culture appear to display a range of phenotypes. Consequently, it has been suggested that as a minimum, MSCs should be defined as cells that express CD73, CD90, and CD105 on the cell surface but which do not express CD45.
Furthermore, MSCs should display plastic adherence and be capable, at least, of adipogenic, chondrogenic and osteogenic differentiation [79]. An exciting feature of MSCs, with potential therapeutic utility, is their possession of immunomodulatory properties while being poorly immunogenic [79]. It is not clear what proportion of so-called MSC preparations are true stem cells. It seems likely that much of the regenerative ability ascribed to MSCs may derive from their immunomodulatory capabilities rather than from their stem cell functions [79]. For example, in a mouse model of spinocerebellar ataxia 2 (SPA2), intravenous administration of human MSCs resulted in improved motor function and preservation of cerebellar Purkinje cells compared to control animals. However, while there was significant engraftment of the human MSCs in the cerebellum of mice, there was no evidence that the MSCs had differentiated and it was concluded that the therapeutic improvements were likely due to paracrine effects rather than stem cell differentiation [80]. Several other studies have also indicated that the therapeutic effects of MSCs are due to paracrine rather than trans-differentiation effects [81].
Promising results have been obtained using mouse mesenchymal stem cells for the development of new therapeutic strategies. In particular, MSCs have been shown to prevent and reverse bleomycin-induced pulmonary fibrosis in C57/Bl6 mice [82]. Moreover, MSCs decreased the number of proinflammatory monocytes. In addition, adipose tissue-derived MSCs significantly reduced the activity of both dextran sulfate sodium- and dinitrobenzene sulfonic acid-induced colitis in mouse models [83]. Furthermore, human MSCs stimulated mucosal regeneration in mouse models of inflammatory bowel disease [84]. In particular, MSCs upregulated cell proliferation in colon epithelium and enhanced CD44-positive cells. With regard to other inflammatory models, human pluripotent MSCs have been shown to suppress allergic inflammation and the development of fibrosis and potentiate the airway regeneration in a mouse asthma model [85]. In addition, antioxidant-stimulated MSCs suppressed inflammation in obese mouse models [86]. Moreover, the severity of fatty liver disease was decreased, since glucose levels were significantly decreased after the treatment with MSCs.
Although both mouse and human ESCs are derived from the ICM of blastocyst stage embryos, they have very different biological properties, and it is now accepted that a linear extrapolation between the two species is just not possible. Mouse ES and iPS cells are positive for SSEA-1, and negative for SSEA-3, SSEA-4, TRA1-60, and TRA1-81. In contrast, human ES and iPS cells are positive for SSEA-3, SSEA-4, TRA1-60, and TRA1-81, but negative for SSEA-1. In culture hES cells do not require LIF whereas mESCs do (Table 3). Mouse EpiSCs seem to share more features with hESCs than mESCs including their flat morphology, dependence on FGF2/Activin signaling, and reduced tolerance to single-cell dissociation by trypsinization. These similarities with mEpiSCs suggest that hESCs may correspond more to the primed pluripotent state rather than to the naive state of mouse ESCs. There are also differentiation differences between mouse and human (Table 3) which may tell us that the well-established protocols developed in the mouse may not translate well to the human system.
Further characterization of hESCs would facilitate the transfer of knowledge from mouse model cells to their human equivalent. Recently, more and more primate and human stem cell lines have been isolated and generated by several laboratories with more than 484 different derivations (as of August 14, 2020) listed in the NIH as eligible for use. With the development of these lines, further insight into human disease may be possible. Interestingly, the processing and subcellular localization of an lncRNA, FAST, important for the pluripotency for human stem cells, is different between human and mouse embryonic stem cells [87].
| Property | mESC | hESC | miPSC | hiPSC |
|---|---|---|---|---|
| Cell Surface Markers | ||||
| SSEA1 | + | - | + | - |
| SSEA3 | - | + | - | + |
| SSEA4 | - | + | - | + |
| TRA1-60 | - | + | - | + |
| TRA1-81 | - | + | - | + |
| Growth factors required | ||||
| LIF | + | - | + | - |
| BMP4 | + | - | + | - |
| bFGF | - | + | - | + |
| TGF/Activin/Nodal | - | + | - | + |
| Wnt | + | + | ? | ? |
| IGF2 | - | + | ? | ? |
| Differentiation ability | ||||
| Embryoid Body | + | + | + | + |
| Teratoma | + | + | + | + |
| Chimera | + | + | - | - |
| Germline Transmission | + | + | - | - |
To progress stem cell-based regenerative medicine to the clinic, suitable animal models are required. Mouse cochlear stem cells have been used as a model for the regenerative therapy of human hearing loss [88, 89]. Martins JP et al reported on the use of lentiviral vectors to transduce both mouse and human enteric nervous system stem cells (ENSCs) with genes encoding fluorescent reporter proteins (eGFP and mCherry) [90]. Following in vitro transduction, the ENSCs were transplanted in the gut of mice. This approach enabled the fate of the transplanted cells to be followed for up to 60 days; the cells were shown to proliferate and incorporate into the endogenous mouse enteric nervous system network [90]. The development of such mouse models provides a way forward for the regenerative treatment of enteric neuropathies and other diseases. Similarly, GFP expression has been used to follow the differentiation of VSELs into cardiomyocytes in a mouse model of myocardial infarction [91].
Applications of stem cell-based animal models are also seen in ophthalmology. A recent study by Tu and Matsuyama have reported the use of retinal multielectrode array to estimate the functions of ganglion cells and field potentials in rods, cones and bipolar neurons in mouse retinas engrafted with retinal tissues generated from stem cells. The study would be advantageous for the development of treatment strategies for retinal degeneration [92]. Furthermore, stem cells might be effectively used for prevention or treatment of retinal pathologies in patients with diabetes. In particular, human bone marrow-derived CD34+ stem cells, which were injected into the retina in streptozotocin-induced diabetic mice (C57BL/6J), were shown to maintain vascular network in retina and affect signalling pathways involved in the development of diabetic retinopathy [93].
In addition, MSCs might be used to decrease inflammation in damaged eye structures. If regeneration mechanisms Negative effects of immune diseases or impaired angiogenesis on the regeneration of corneal epithelium might cause continuous epithelial damage. Extracellular vesicles, which are produced by stem cells and contain bioactive compounds, showed promising effects [94]. The study by Tao et al. has shown that extracellular vesicles derived from MSCs had anti-inflammatory and anti-apoptotic effects in corneal epithelium. Thus, MSC-derived vesicles improved corneal wound healing in mice and might be used as an alternative treatment approach.
Gastroenterologists may also benefit from stem cell-based therapeutic approaches. In particular, the beneficial effects of MSC and bone marrow-derived stem cells administration to mice with chemically induced cirrhosis have been observed by Watanabe et al [95]. Stem cell-based therapy stimulated hepatocyte proliferation and decreased the levels of liver enzymes in the blood. Moreover, stem cell therapy caused the infiltration by macrophages and neutrophils leading to the fibrosis suppression and activation of regenerative process in the liver. Another study has demonstrated that MSCs decrease the levels of alanine aminotransferase and fibrosis in mice [96]. Especially, significant therapeutic effects were detected when using MSCs pre-cultured in hypoxic conditions. Hypoxia-affected MSCs induced anti-inflammatory macrophage phenotype via activation of prostaglandin E2. Thus, MSCs were suggested to be potentially useful for the development of the new treatment strategies for liver cirrhosis.
Multiple studies have reported that stem cells upregulate liver regeneration. In addition, stem cell derived hepatocyte-like cells have been demonstrated to diminish hepatocyte damage in mice models for fibrosis [97]. The results of cell therapy were significantly improved hepatocyte-like cells together with umbilical vein endothelial cells and MSCs. In line with these findings, human liver-derived stem cells had high hepatogenic function, since transplanted cells migrated into the ischemia-damaged liver in a mouse model and induced liver regeneration [98].
With regard to the regulation of MSCs functions, Cho and Park have recently shown that ferulic acid positively regulates self-renewal of embryonic stem cells and MSCs [99]. Notably, ferulic acid upregulated NANOG mRNA expression in ESCs and MSCs.
Human intestinal organoids (HIOs) derived from stem cells is also a valuable tool to study intestinal morphogenesis. Bouffi et al reported HIOs containing immune cells were developed by engrafting them under the kidney capsule of mice with a humanized immune system. Human immune cells migrated to the mucosa and built cellular aggregates resembling lymphoid follicles. They also observed the secretion of IgA antibodies, hence indicative of immune activation, in the HIOs after experimental microbial infection [100].
To study human intestinal-immune interactions during development, HIOs containing immune cell subsets were developed by transplanting HIOs under the kidney capsule of mice with a humanized immune system [100]. In the transplanted organoids, human immune cells infiltrated the mucosa and produced cellular clusters similar to human lymphoid follicles. Furthermore, microbial exposure induced the expansion of epithelial microfold cells and increased production of IgA antibodies in the lumen of the interstinal organoids.
Intestinal organoids were also applied to study tissue regeneration [101]. Senescence-associated secretory phenotype factors produced by senescent fibroblasts were demonstrated to suppress stem cell differentiation and crypt formation. N-terminal domain of Ptk7 was found to stimulate non-canonical Wnt/Ca2+ signaling through FZD7 and consequently induce nuclear translocation of YAP and expression of YAP/TEAD target genes, which in turn inhibit symmetry breaking and stem cell differentiation.
Studies using a mouse model of systemic lupus erythematosus (SLE) have showed that immunoregulatory effects of adipose-derived MSCs can be potentiated by metformin [102]. The authors demonstrated metformin-mediated suppression of p-STAT1, p-STAT3, Il-10 and TGF. Thus, metformin might be useful for the development of novel therapeutic strategies for lupus patients.
Also, another study has evaluated the beneficial effects of MSCs for patients with SLE by treating NZBWF1 mice, an experimental lupus animal model, with MSCs [103]. Treatment with MSCs significantly decreased proteinury and anti-dsDNA antibody level, affected the production of inflammatory cytokines and suppressed NF-κB signaling. In addition, transplantation of MSCs into MRL/lpr mice significantly decreased the degree of alopecia, ulceration, proteinuria and the level of anti-dsDNA antibodies [104].
The effects of MSCs on the development of dry eyes through the inhibition of autophagy markers were studied in a Sjogren’s syndrome mouse model [105]. According to the report, NOD/ShiLtJ female mice with developed keratoconjunctivitis underwent either subconjunctival or lacrimal gland injections of human MSCs. The study showed reduction of B cell marker B220 and proinflammatory cytokines in lacrimal glands. Moreover, the treatment with human MSCs decreased the expression of autophagy markers ATG5 and LC3B-II.
To study profibrotic interactions between T cells and mesenchymal cells in a scleroderma model, humanized MISTRG6 mice engrafted with unmatched allogeneic HSCs, were transplanted with normal and scleroderma skin from a patient with pansclerotic morphea [106]. The analysis has shown that HSC engraftment suppressed the intensity of fibrosis and proliferation of T cells. Moreover, decreased fibrosis was associated with diminished expression of IL-6 by mesenchymal cells. Furthermore, the study has shown that IL-6 signaling induced by the interaction between CD4 T cell-derived soluble IL-6 receptor and fibroblast-produced IL-6 activated extracellular matrix gene expression.
Endothelial progenitor cells might be used to stimulate myocardial recovery in ischemic heart disease. In vitro generated human endothelial colony-forming cells were injected into the ischemic heart region in SCID/beige mice, which served as a model of ischemic heart disease [107]. Myocardial function was significantly improved by the transplantation of the stem cells. Notably, the regeneration of cardiomyocytes was stimulated by the intensified local neovascularization and the activity of Sca1+ myocardial stem cells. In addition, human neural stem cells were shown to prevent stroke development in aged stroke mice [108]. Moreover, stem cell transplantation suppressed the production of proinflammatory molecules, such as TNFα and IL-6.
Cardiomyopathy caused by mutations in LMNA, a gene encoding A-type lamins, was investigated using mouse pluripotent embryonic stem cells (ESCs) and a mouse model carrying the p.H222P Lmna mutation [109]. The authors detected the impairment of cardiac differentiation of mutated ESCs and dilatation of embryonic hearts at E13.5 in the experimental animals. In addition, the inhibition of LSD1, which specifically demethylated H3K4me1, prevented cardiomyopathy in both offspring and adult animals, making LSD1 a suitable molecular target to prevent dilated cardiomyopathy.
As hair-follicle-associated pluripotent (HAP) stem cells undergo the differentiation into both glia and neurons, the abilities of these cells to repair injuries of the nervous system have been assessed using two mouse models [110]. In their recent study, Obava et al. implanted HAP cells from C57BL/6J mice into the injured brain of C57BL/6J or nude mice and showed that implanted stem cells differentiated into neurons and microglia in the ICH site of nude mice and inhibited astrocyte and microglia infiltration in C57BL/6J mice. Overall, the study suggested that HAP cells may potentially repair intracerebral hemorrhage.
To develop new methods of cell therapy for Parkinson's disease (PD), stem cells-derived human midbrain organoids 3-D (hMOs) were transplanted as tissue pieces into the striatum of naïve immunodeficient mouse brains [111]. The analysis was performed 12 weeks post-transplantation of hMOs and the results showed that 14.11% of the engrafted cells expressed TH+ and most of these cells were co-labeled with GIRK2+, demonstrating the viability and maturation of A9 mDA neurons in the striatum of PD mice. Transplantation of hMOs reversed motor function and indicated the development of connections with natural brain regions.
Potential benefits of hematopoietic stem and progenitor cells (HSPCs) for treatment of neuronal ceroid lipofuscinosis type 1 (CLN1) neurodegenerative disorder caused by palmitoyl-protein thioesterase-1 (PPT1) deficiency [112]. Transplantation of HSPCs decreased the severity of CLN1 symptoms in the mouse model of CLN1. Furthermore, combined transplantation of PPT1-overexpressing transduced HSPCs intravenously and via intracerebroventricular route showed in a stable therapeutic effect.
In the past 30 years stem cell research has advanced exponentially. With the help of mouse models, techniques for isolation, culture, and transplantation have become established. There is a current lack of data on the epigenetic status of ESCs, and the possibility that DNA methylation, for example, is altered during culture needs to be determined. The differences between mouse models and humans need to be further investigated if they will be used in the treatment of human diseases. Translation into the human, therefore, may take a little longer to become routine in a clinical setting, as there are some factors which still need to be overcome such as the problem of iPSCs eliciting an immune response in the recipient and the possibility of tumor formation. The generation of patient-specific iPSCs, however, will have a significant impact on human disease and regenerative medicine though it is important to establish strategies of reprogramming that do not alter the genetic make-up of the cell. With the fast advancement of these technologies, it will not be surprising if these obstacles are overcome very soon.
- Martin G. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-8 pubmed
- Evans M, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-6 pubmed
- Ward C, Stern P, Willington M, Flenniken A. Efficient germline transmission of mouse embryonic stem cells grown in synthetic serum in the absence of a fibroblast feeder layer. Lab Invest. 2002;82:1765-7 pubmed
- Schoonjans L, Kreemers V, Danloy S, Moreadith R, Laroche Y, Collen D. Improved generation of germline-competent embryonic stem cell lines from inbred mouse strains. Stem Cells. 2003;21:90-7 pubmed
- Ying Q, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281-92 pubmed
- Kashyap V, Rezende N, Scotland K, Shaffer S, Persson J, Gudas L, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093-108 pubmed publisher
- Prelle K, Zink N, Wolf E. Pluripotent stem cells--model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 2002;31:169-86 pubmed
- Tesar P, Chenoweth J, Brook F, Davies T, Evans E, Mack D, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196-9 pubmed
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-76 pubmed
- Hanna J, Wernig M, Markoulaki S, Sun C, Meissner A, Cassady J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920-3 pubmed
- Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-7 pubmed
- Al Hajj M, Wicha M, Benito Hernández A, Morrison S, Clarke M. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-8 pubmed
- Patrawala L, Calhoun T, Schneider Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696-708 pubmed
- Ricci Vitiani L, Lombardi D, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-5 pubmed
- Singh S, Clarke I, Terasaki M, Bonn V, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-8 pubmed
- Patrawala L, Calhoun Davis T, Schneider Broussard R, Tang D. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796-805 pubmed
- Cho R, Wang X, Diehn M, Shedden K, Chen G, Sherlock G, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364-71 pubmed
- Zhou Z, Flesken Nikitin A, Nikitin A. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 2007;67:5683-90 pubmed
- Shultz L, Lyons B, Burzenski L, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477-89 pubmed
- Shultz L, Schweitzer P, Christianson S, Gott B, Schweitzer I, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180-91 pubmed
- Mombaerts P, Iacomini J, Johnson R, Herrup K, Tonegawa S, Papaioannou V. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869-77 pubmed
- Brinster R, Zimmermann J. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298-302 pubmed
- Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115:1855-61 pubmed
- Ogawa T, Dobrinski I, Avarbock M, Brinster R. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29-34 pubmed
- Kucia M, Reca R, Campbell F, Zuba Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857-69 pubmed
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz Masiuk M, Moldenhawer S, Zuba Surma E, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297-303 pubmed
- Bhartiya D, Shaikh A, Anand S, Patel H, Kapoor S, Sriraman K, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016;23:41-76 pubmed
- Wang H, Tian Y, Li X, Yang M, Yan Y. Amniotic mesenchymal stem cells derived hepatocyte-like cells attenuated liver fibrosis more efficiently by mixed-cell transplantation. Int J Physiol Pathophysiol Pharmacol. 2020;12:11-24 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- gene
- human BMP4
- human FGF-2
- human IGF2
- human LIF
- human NODAL
- human WNT1
- mouse Akna
- mouse Arhgef2
- mouse Aspm
- mouse Bmi1
- mouse Cdkn2a
- mouse Dct
- mouse Dock7
- mouse Fgf13
- mouse Fgfr1
- mouse Fgfr2
- mouse Fubp1
- mouse Fzd7
- mouse Hoxb4
- mouse Kit
- mouse Lef1
- mouse Lrp6
- mouse Notch1
- mouse Numb
- mouse Numbl
- mouse Rab10
- mouse Sox5
- mouse Tead3
- mouse Tgfb2
- mouse Vangl2
- mouse Wnt3a
- mouse Wnt7a
- mouse Zfp36l2
method- 3D Cell Culture: A Review
- Alzheimer’s Disease: Genes
- Behavioral Phenotyping in Rats and Mice
- Hematopoietic Stem Cells and Hematopoiesis
- Huntington's Disease Animal Models
- Laboratory Animals Companies
- Laboratory Mice and Rats
- Mice and Rats: Housing Temperature and Handling
- Stem Cell Markers
- Stem Cells
- Xenopus laevis as a Model System

