An overview of imaging techniques for RNA molecules.
The ability to visualize specific RNA in situ is essential to understand the regulatory mechanisms of gene expression, RNA processing, and splicing. An improved understanding of these approaches is also necessary in order to identify new drug targets, as well as to create particular gene expression patterns. A variety of protocols have been developed to measure the expression levels of various RNA species, using either fixed-cell or live-cell imaging methods. Despite the fact that much of the knowledge about the spatial distribution of RNA originated from fixed-cell imaging, live-cell imaging strategies provided better possibilities along with added capabilities for real-time monitoring of RNA transport into living cells. These live-cell imaging studies have dramatically improved our understanding of the role of RNA dynamics in various cellular functions. Nevertheless, a range of newer experimental approaches and/or protocols in RNA imaging technologies are continuously evolving. This review summarizes the state of the art achieved by RNA imaging technologies and its impact on RNA biology studies.
The process of protein synthesis initiates from individual DNA molecules within the living cell. Although, the information for protein synthesis is stored in DNA, however, for achieving successful gene expression several key classes of RNA molecules works in coordination. Three kinds of RNAs, messenger RNA (mRNA), transfer RNA (tRNA) & ribosomal RNA (rRNA) carry out their specific tasks, leading the accurate synthesis of proteins, which is critical to the proper functioning of cells and organisms. Non-Coding RNA transcripts participate in catalytic, structural and regulatory functions, whereas mRNA defines the gene expression.
RNA molecules are responsible for a plethora of functions in living cells; an RNA transcript in its journey from biogenesis to degradation undergoes to several changes within the cell. Since each subcellular compartment of the cell provides a specific environment for RNA transcript, it is important to understand how processing of the RNA at different levels within the cell affects and regulates the gene expression. These quests have led to the discovery and development of various techniques that permits the tracking of RNA inside the cells.
The methods for determining and monitoring the spatial localization, temporal regulation of RNA and its underlying mechanism can be divided into two main categories: fixed-cell imaging and live-cell imaging. In this review, we will discuss some of the most common RNA imaging protocols.
Fixed-cell RNA imaging is primarily based on in situ hybridization (ISH), a technique introduced in 1969, by which precise localization of a nucleic acid segment in a fixed cell is determined by using labeled oligonucleotide probes [3]. Probes can be labeled in vitro with a fluorophore where the site of hybridization can be detected directly (FISH). In contrast, in the indirect labeling, an additional enzymatic or immunological system is required to visualize the hybridized probe. Indirect labeling is not a preferred method of choice for RNA visualization because of the problems associated with the use of an additional system. As such, ISH techniques provide important clues about expression and subcellular localization of a gene and a number of studies have applied ISH to study localization and quantification of specific nucleic acid sequences in the individual cells and tissues [1, 4-6].
The global RNA level (vs. specific RNA) can be easily detected through 5-ethynyl uridine and click chemistry such as Thermo Fisher Click-iT™ RNA Alexa Fluor™ 594 Imaging Kit [7].
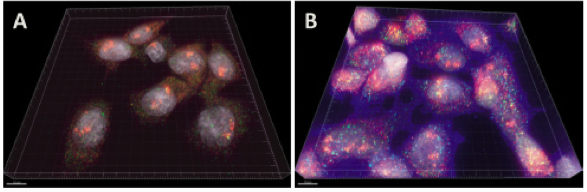
FISH provides a powerful tool for identifying the location of RNA in fixed cells. Figure 1 shows a schematic diagram illustrating FISH. A number of studies have applied FISH to determine gene expression in different cellular systems [1, 5]. FISH has been used to examine the localization of beta-actin mRNA in chicken embryo fibroblasts and lac Z transcripts in yeast cells [8]. Recently RNA FISH was used on frozen slices of mouse intestine by van Oudenaarden to show the distribution of three types of stem cell markers transcripts within crypts [9]. Since the introduction of FISH in the 1980s, numerous variations of FISH methodologies have been developed for detecting RNA in cells, which have significantly improved the sensitivity, specificity, and speed of FISH. One such variant is single molecule FISH (smFISH), developed by Singer and colleagues [10]. In smFISH multiple fluorophore-labeled probes are hybridized to a single mRNA. In recent years, the smFISH probe design has improved significantly and many newer probes with increased sensitivity have been developed for enhanced detection of single RNA molecules. As a result, smFISH is the considered the benchmark technique for quantifying individual transcripts in the nucleus. Commercial probes (Stellaris) are available [11, 12]. Figure 2 shows smFISH and ImmunoFISH (a combined smFISH with immunohistochemistry) of mRNA particles and activated signaling proteins [1]. Tyagi and co-workers modified smFISH, by using multiple, short probes (20-nucleotide) labeled with single fluorophores [13, 14]. The advantage of these probes is that they are cheaper, easy to make and can penetrate cells more easily. These short singly labeled probes pooled into sets of up to 48 individual probes are commercially available as a ‘Stellaris FISH Probes’ (Biosearch Technologies). de Morree A et al hybridized FACS-isolated muscle stem cells with a library of forty-eight Stellaris RNA-FISH probes against Pax3 to compare the expression of Pax3 RNA isoforms between diaphragm and limb muscle stem cells [15]. Hafner AS et al used Stellaris probes from LGC Bioresearch to locate specific mRNA molecules in synaptosomes and cultured neurons to study the local protein synthesis in neuronal pre- and postsynaptic compartments [16]. FISH probes were used for the simultaneous imaging of multiple primary, mature mRNA, immature noncoding RNA gene products, and RNA variants in fixed mammalian cells [17]. RNAscope was developed to achieve single molecule RNA biomarker detection for both regular fixed cells and tissues, for example heart tissues [18], and more significantly, for clinically important formalin-fixed, paraffin-embedded tissues [19]. RNAscope can be combined with immunostaining to examine both mRNA and protein expression, for example, [20]. smFISH has also been adapted for use in organoids [21] and whole mount 3D embryos [22], highlighting its application for studying complex biological samples whilst preserving the morphology of the system.
Further recent advances of smFISH and its derivative have allowed the process to reach larger scales by sequential hybridisation of FISH probes [23]. This technique, often called seqFISH has been utilised to analyse transcriptional profiles in biological systems. SeqFISH was used to analyse over 200 genes in hippocampal tissues [24], where distinct transcriptional profiles were mapped in different substructures. SeqFISH has also been used in detection of the nascent transcriptome as well as dynamic nuclear organisation in single cells [25]. More recently, an adaptation named seqFISH+ has been used to detect over 10,000 genes in single cells of the mouse brain [26]. SeqFISH+, published in Nature in 2019, utilises super-resolution imaging, allowing robust detection of a huge number of mRNAs in complex tissues. Thus, in recent years, smFISH has reached the genomic scale.
In situ hybridization chain reaction (HCR) can be multiplexed and quantitative [27]. A Marconi et al detected Col2a1, Sox9, Sox5 and Sox6 mRNAs on paraffin sections from skate Leucoraja erinacea with HCR [28].
| Supplier | Products | Sample References |
|---|---|---|
| Roche | digoxigenin RNA labeling kit | zebrafish whole-mount [29], frozen mouse brain sections [30] |
| T3, T7 or SP6 RNA polymerases | 35S-label probes on frozen finch brain sections [31] | |
| Advanced Cell Technologies / Bio-Techne | RNAscope | frozen fixed mouse brains [32, 33] ; frozen fixed olfactory bulbs and brain [34], PFA-fixed mouse brain [35] ; formalin-fixed paraffin-embedded macaque lung [36] |
| Thermo Fisher | QuantiGene ViewRNA miRNA ISH Cell Assay | cultured neurons and frozen hippocampal sections [37] |
| QuantiGene ViewRNA ISH Cell Assay | ViewRNA probe used for PLK1 mRNA FISH [38] ; for Cd3e, Cd19, Cd14 and Rpl13a [39] | |
| FISH Tag DNA Kit | probes labeled with Alexa Fluor 488, 555, and 647 dyes for 3D FISH on S2R+ Drosophila cells [40] |
Nevertheless, FISH imaging technique is conducted on a fixed sample, therefore, it provides limited information about the real-time intracellular RNA dynamics. Other limitations are the laborious and time-consuming nature of the assay, the difficulty that involves the analysis of the images and variations due to the handling of different batches. The chemicals used for dehydration and fixation could also affect the signal level and alter the integrity of organelles, hampering any conclusions about spatial changes in organelles or compartments [41]. However, with continuing advances in technologies, such as seqFISH, and super-resolution imaging, smFISH continues to generate exciting data that improve our understanding of the transcriptome in time and space. Table 1 lists the commonly used reagents for FISH studies.
| Live Cell- RNA Imaging Techniques | Details | Limitations | References |
|---|---|---|---|
| Tagged Linear Oligonucleotide Probes (ODN) | Single fluorescent labeled linear oligonucleotide probe developed for RNA tracking and localization studies in living cells. | Lack of specificity, and limited ability to distinguish the background from true signal. | [42] |
| Linear Fluorescence Resonance Energy Transfer (FRET) Probes | Novel way to increase the signal-to-noise ratio and improved detection specificity by introducing two linear probes (FRET donor and acceptor fluorophores). | FRET still have a high background signal due to direct excitation of the acceptor and emission detection of the donor fluorescence. | [42] |
| Molecular Beacons (MB) | Sensitive probes with improved specificity and higher signal to background ratio. MB has the capability to discriminate between perfectly matched and single mismatched targets. | Requires extensive optimization for melting temperature, stem length, loop length, and hybridization. | [43, 44] |
| Genetically Encoded RNA Reporters (MS2CP, λN22, PP7, eIF4A, PUMILIO1, BgIG, U1Ap, HTLV-1Rex, TAR-TAR, REV-RRE, PFC System) | Genetically encoded probes bypasses the various limitations associated with the use of fluorescent probes for RNA detection in living cells. | Low sensitivity due to high background fluorescence developed from unbound fluorescent protein. | [45] |
| RNA-Dye Interaction Systems (Spinach, Spinach2, Broccoli, Mango, IMAGE, Malachite green, SRB-2) | The RNA-dye based interaction systems use exogenous small organic fluorescence dye to label RNA. These fluorophores exhibit lower signal-to-background ratio fluorescence. | Limited availability of suitable fluorogenic dye. | [2], [46-48] |
A key feature of complex tissue environments that is not detected by standard single cell RNA sequencing technologies is the spatial organisation of the cells within. However, recent advances in the field of spatial transcriptomics have begun to shed light on this layer of regulation [49-51]. One of the most recent and high-resolution advances addressing spatial transcriptomics is of Slide-seq, a method which allows the user to study the transcriptome of a tissue sample in situ, thus retaining spatial information at a high resolution [52]. Published in Science in 2019, Slide-seq represents a powerful tool providing a unique view into the molecular signatures of cellular organisation.
Slide-seq begins with an array of DNA-barcoded beads, which are hybridised to a slide in a random, monolayer arrangement. The layout of these barcodes on the slide is uncovered by SOLiD (sequencing by oligonucleotide ligation and detection), which relies on emulsion PCR [53]. Cryosections of the tissue of interest are then melted onto the slide with the hybridised barcodes. The mRNA from the tissue then becomes trapped onto the beads through RNA hybridisation. The sample is then subject to reverse transcription, before the tissue is digested and the sequencing library is prepared [52].
Because the arrangement of the barcoded beads on the slide is known, specially tailored software can be employed to map the location of the sequenced mRNAs to a 10µm resolution, which corresponds to single-cell scale. This gives researchers a map showing the location of expressed genes in their tissue. Slide-seq detects approximately 400 unique transcripts in each cell, meaning if multiple sections are analysed, even rare transcription events can be observed [52].
Slide-seq can be applied to a range of tissues. To demonstrate this, the authors were able to analyse fine-range structures in both mouse and human brain tissues, and in liver cells, finding that their observations were consistent with RNA-sequencing data. This method has exciting prospects for translational medicine, allowing researchers to map gene expression changes in complex tissues in response to treatments, disease or other perturbations. Slide-seq is particularly exciting for researchers studying heterogeneous tissues in which spatial information is missing and vital, such as in tumours, the brain, and in the gastrointestinal tract.
RNA imaging in living cells is of paramount importance in biological and clinical research as it provides comprehensive information on the expression, localization, degradation, storage and regulation of diverse classes of cellular RNAs molecules. A variety of techniques for imaging RNA in living cells have been developed in past years. These different strategies provided important and accurate insight into the role RNA plays in dictating cell behavior and has added dynamic dimensions to the field of RNA biology. Live-cell RNA imaging system not only allows researchers to study in detail the complete cellular route of an RNA transcript from its biosynthesis to degradation, but also it eliminates the many drawbacks associated with the handling of RNA in fixed cells, for example, the use of fixative agents and washing. In the future, live-cell RNA imaging would be of significant value to decipher the role of genetic processing during cellular function and diseases [54].
Multiple approaches are now being used for RNA imaging in living cells (Table 2). A recent innovation, termed CRISPR LiveFISH, is the use of catalytically-deactivated Cas13d and fluorescent gRNAs for real-time RNA imaging [55]. These techniques include, but are not limited to direct labeling of RNA by incorporation of a fluorescent nucleotide, genetically encoded RNA reporters and RNA-dye interaction systems. In this section, we will briefly discuss some of the most commonly used live cell RNA visualization techniques.
Several types of probes have been devised to detect RNA molecules.
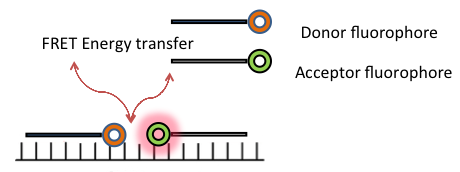
Visualization of endogenous RNA by labeled linear oligonucleotide (ODN) probe is one of the simplest approaches for RNA tracking and localization in living cells. However, this approach has several limitations including, lack of specificity, possibilities of rapid accumulation in the nucleus after delivery using microinjection and limited ability to distinguish the background from true signal [42]. These shortcomings have prevented the applications of linear ODN probes in live cell RNA imaging. In contrast, the dual linear probe approach such as, fluorescence resonance energy transfer (FRET) displays a significant improvement in selectivity and signal to background ratio. FRET methodology consists of two oligonucleotides, labeled with two different fluorophores (acceptor and donor fluorophores), which hybridizes to neighbouring regions of target RNA. The transfer of energy from donor to acceptor fluorophore, when excited by an external light source, produces a FRET signal.
Although duel linear FRET probes demonstrate improved sensitivity and higher signal to background ratio, they still have a number of problems in their specificity, which has limited their use for a wide variety of biological and disease studies [42]. Figure 3 shows a schematic diagram illustrating FRET.
Molecular beacons (MB) developed by Sanjay Tyagi and Fred Russell Kramer, are hairpin shaped oligonucleotides hybridization probes, designed with a fluorophore on one end and a quencher on the other [56]. A typical molecular beacon has four essential parts: a loop, a stem, a fluorophore (fluorescent dye), and a quencher. The loop usually consists of 15–25 nucleotides and is complementary to the target sequence. The stem, formed by two short-arm sequences which are complementary to one another, is typically 4–6 bases long. The fluorophore is covalently linked to one end of the oligonucleotide. The quencher is a non-fluorescent moiety covalently linked to the other end of the oligonucleotide. In the absence of the complementary target, the arms form a stem-loop hairpin structure, keeping the two ends in close contact and the beacon dark. Once hybridized with the target nucleic acid, it forms a relatively strong probe–target hybrid that is longer and more stable than the stem hybrid. This causes the two ends of the molecular beacon to get physically separated and undergo a conformational reorganization allowing a fluorescent signal to be emitted upon excitation. [56]. Figure 4 shows a schematic diagram illustrating molecular beacons.
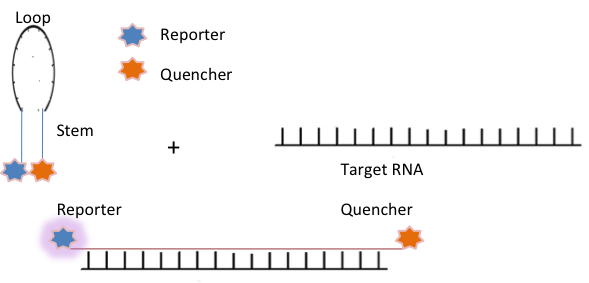
Molecular beacons contain sensitive probes with improved specificity and higher signal to background ratio. They have the capability to discriminate between perfectly matched and single mismatched targets. They have been used successfully in a wide variety of biological and biomedical applications, including real-time monitoring of PCR, SNPs detection by real-time PCR, genotyping and mutation detection, monitoring viral infection, detection of multiple analytes and cancer cells and tracking of RNA expression, transport, and localization in living cells [43, 44, 56, 57]. For RNA imaging in living cells, an appropriate design of the MB stem sequence is required for achieving enhanced specificity and sensitivity. A longer stem provides more stable hairpin conformation with a higher signal-to-background ratio, however, increasing stem strength reduces the capacity of the hybridization [54]. To attain the higher specificity, it is necessary to determine a fine balance between selectivity and hybridization rate for any given assay. As such, the use of MB for live cell RNA imaging requires extensive optimization in terms of melting temperature, stem length, loop length, and hybridization [58]. Moreover, problems in cell delivery without perturbing cellular process have indeed limited their use in live cell RNA imaging [54]. Nevertheless, MB technology has been significantly improved in last two decades, by collaborative efforts of many researchers, thus expanding their application in various fields of disease diagnosis, including bioimaging, gene therapy and drug delivery [59, 60]. More probe variants with higher specificity and faster hybridization kinetics have been developed, including tentacles probe, photoactivatable probes; or multiply labeled tetra(poly)valent RNA imaging probes (MTRIP) [61], FIT (forced intercalation) or ECHO (exciton-controlled hybridization-sensitive oligonucleotide) probes [62]. Recently, new improved approaches for imaging of endogenous miRNAs based on Quantum dots, single cell detection of mRNAs and real-time imaging of single engineered RNA transcripts in living cells have also been developed [63-65].
Genetically encoded probes are important tools in RNA imaging as they bypass the various limitations associated with the use of fluorescent probes for RNA detection in living cells. For instance, they can be produced by cellular machinery and can be expressed directly in the cells after a DNA sequence is introduced [66]. Here, we will highlight the most widely used genetically encoded RNA reporter systems.
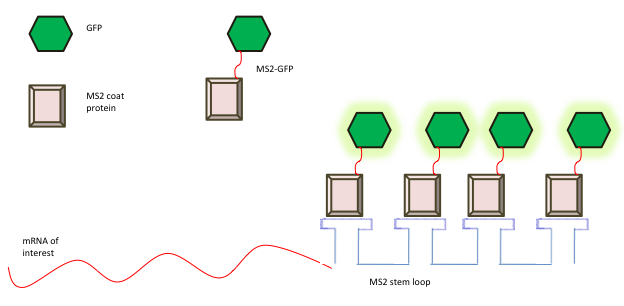
A substantial advancement in mRNA imaging in live cells was the use of direct RNA-binding proteins (RBPs) tagged with optical reporters such as green fluorescent protein (GFP) [13]. MS2CP was the first genetically encoded RNA imaging system described by Bertrand et al in 1998 [67]. The MS2 bacteriophage system is based on the direct fluorescent tagging of mRNAs using the MS2 bacteriophage. The coat protein of the bacteriophage MS2 binds tightly to a unique RNA hairpin sequence (MS2-binding site (MBS)) in viral RNA to form the viral capsid structure. The feasibility of this high-affinity interaction to study the real-time dynamics of RNA was first demonstrated by Singer and co-workers by constructing a two plasmid system in yeast. On one plasmid, the GFP sequence was fused to a gene that encodes the coat protein of the bacterial phage MS2 (GFP-MS2), along with an engineered nuclear localization signal. Therefore, if GFP-MS2 is not complexed with RNA, it would be restricted to the nucleus. On the second plasmid, six MS2-binding sites of the coat protein of bacteriophage MS2, each consisting of a 19-nucleotide RNA stem-loop was inserted into a reporter mRNA to have increased sensitivity of detection [67]. Multimerisation of the MS2-binding sites and co-expression of MS2 coat protein fused to GFP (GFP-MS2) enables the visualization of mRNA dynamics and localization in living cells [68]. Figure 5 shows a schematic diagram illustrating the MS2-GFP probe for live cell RNA detection. The MS2-GFP for coating RNA transcripts with fluorescent proteins has provided a great deal of insight into RNA processing, localization, and transport, and is most commonly used method for studying RNA dynamics in various model organisms [69-75]. Recently, Singer and colleagues have established the MS2 system in in vivo in mice. They have developed transgenic beta-actin-MBS mouse model (Act-MBS mouse), in which 24 MBS motifs in a row were integrated to a beta-actin chromosomal gene in its native chromosomal location. Neurons and primary fibroblasts from the Act-MBS mouse were used for live cell imaging. However, this approach still needs further validation to other genes which are not as abundant in cells as beta-actin. Nevertheless, the generation of transgenic Act-MBS mouse has opened a new window of possibilities for in vivo RNA visualization [76].
Alternative RNA imaging systems with other bacteriophage proteins, based on the same principle as MS2-GFP have also been developed. The λN22 system was developed by Daigle and Ellenberg and was first described in mammalian cells [77]. λN22 is a 22 amino acid peptide of N protein of bacteriophage that tightly and specifically binds to box B, a short motif of 15nt to its cognate RNA. The small size of the RNA binding protein and the short size of the aptamer is a significant advantage of the system as it reduces the impact on the cellular properties of the transcript under investigation.
The λN22 system is the second most commonly used systems after MS2CP, and it has yielded valuable information on the dynamic behavior of mRNAs and their transport partners in various cellular processes in bacterial, fungal, plant and mammalian cells [45, 78].
The PP7 system developed by Singer and colleagues is based on bacteriophage PP7, an RNA phage of Pseudomonas [79]. They used the PP7 system to measure the dynamics of nascent RNA—including transcription initiation, elongation, and termination at an active yeast locus. The coat protein of bacteriophage PP7 binds to its cognate RNA structure with very high affinity [80]. Recently, the bacteriophage PP7 RNA-labeling system was used in Saccharomyces cerevisiae to follow single-particle mRNP export events with high spatial precision and temporal resolution [81]. The PP7 system was also combined with the MS2 system to form a fluorescence complementation labeling system [82]. Bacteriophage MS2/PP7 coat proteins and their respective RNA binding motifs were used, to bring the two halves of a split-FP together to image single reporter mRNAs without background fluorescence [82].
The eIF4A system developed in 2007 by Broude and colleagues is based on the mouse version of the eukaryotic initiation factor 4A (eIF4A) [83, 84]. eIF4A is a dumbbell shaped protein, which contains two RNA-binding domains, each of which binds with strong affinity to one side of the large 58 nt aptamer. eIF4A system was then divided into two parts and each part was fused with half of a green fluorescent protein [85]. A functional green fluorescent protein is formed when the two GFP halves are brought in close proximity. Other related approaches have been described such, TAT-TAR and REV-RRE systems [86], HTLV-1 Rex system [87], U1Ap system [88, 89] and BglG system [90].
PUMILIO1 developed by Yoshio Umezawa and colleagues in 2007, consist of two RNA-binding domains of human PUMILIO1, a unique RNA-binding protein, each fused to split fragments of a fluorescent protein capable of restoring fluorescence upon binding to a target RNA [91]. Beta-actin mRNA in mammalian cells was successfully visualized using fluorescent protein tag Pumilio [92]. Pumilio-based reporter system has also been used to track viral RNAs in plants [93].
A downside of the MS2-GFP system which limits its sensitivity is high background fluorescence coming from unbound fluorescent protein. Protein complementation assay (PCA), to improve the signal-to-background ratio of fluorescent protein–based imaging of RNA is, based on the concept of protein fragment complementation (PFC) [83].
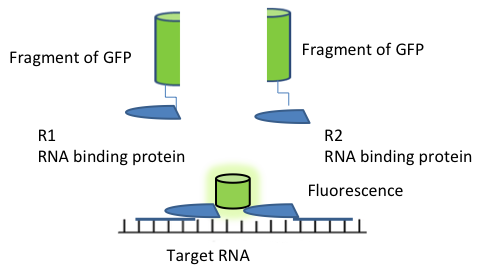
In PCA, a fluorescent protein (FP) is split into two non-fluorescent fragments, which are fused to two different RNA binding proteins (RBP). These two RBPs binds strongly and specifically to two different RNA motifs, placed in adjacent locations in the target mRNA. The two non-fluorescent fragments do not bind to each, but when the RBPs bind to their corresponding motifs that are positioned adjacent to each other in the RNA, the two non-fluorescent fragments are also brought close to each other to form a complete FP, which results in activation of an optical signal [83, 94]. Figure 6 shows a schematic diagram illustrating the PFC system.
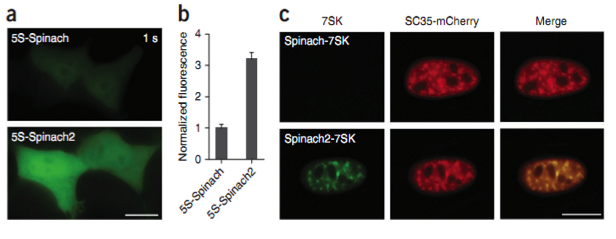
The RNA-Dye based interaction systems use exogenous small organic fluorescence dye to label RNA in living cells as a replacement of fluorescent protein (GFP). The specifically designed RNA sequence (aptamer) binds to a fluorophore and activate the fluorescence [95]. These fluorophores exhibit lower signal-to-background ratio fluorescence when incubated with cells. The first RNA-fluorophore complex developed for RNA imaging termed ‘Spinach’ and was developed by Jaffrey and colleagues in 2011 [96]. Spinach is composed of 80 nt long RNA aptamer, that binds and switches on the fluorescence of GFP-like fluorophore HBI. HBI is an organic dye, 4-hydroxybenzylidene imidazolinone which corresponds to the fragment of GFP responsible for its fluorescence. However, analysis of spinach showed that it exhibits an overall dimmer fluorescence than expected. Researchers later discovered that spinach is not properly folded in cells maintained at 370C as it is thermally unstable due to its low Tm and also requires high magnesium ion concentration which is normally not found inside the cells. To overcome this problem more thermally stable, brighter enhanced version of spinach named as spinach 2, was designed by the same group [97]. Figure 7 shows 5S-Spinach2 is brighter than 5S-Spinach in mammalian cells [2]. Several other RNA aptamer fluorophores like Brocolli, Baby Spinach, Mango and IMAGE (Intracellular MultiAptamer GEnetic) were also developed to tag RNA and to follow its subcellular localization [96, 97]. Interestingly, Broccoli was found more thermostable than spinach1 and spinach 2 and with lesser magnesium dependencies. Similarly, RNA Mango, a high affinity RNA aptamer which binds a series of thiazole orange (fluorophore) derivatives exhibits better fluorescent efficiency than RNA Spinach. Autour A et al developed three new high-affinity RNA Mango fluorogenic aptamers [98]. Nevertheless, Spinach was the first small molecules based RNA imaging system successfully used in living cells [2]. Recently, IMAGE was also developed to follow RNA transcription in live yeast cells [46]. Overall, the use of RNA-dye interaction systems for live cell imaging is still in the developing stages and has a much wider potential for improvement, which in future will help researchers for detailed analysis of RNA dynamics.
An SRB-2 system introduced by Sunbul and Jaschke, in 2014, uses dinitroaniline, an efficient general contact quencher, 54 nt SRB-2 aptamer (sulforhodamine B binding aptamer) and Sulforhodamine B (SR) as the dye [47, 48]. Dinitroaniline when conjugated to fluorophores, quenches their fluorescence by forming a non-fluorescent intramolecular complex. RNA of interest here is fused to the quencher binding aptamer (SRB-2 aptamer). The binding of SRB-2 RNA aptamer to the contact-quenched fluorophore-dinitroaniline conjugates destroys the fluorophore-quencher complex, leading to an increase in fluorescence [47, 48].
in situ visualization of RNA is becoming increasingly important to analyze gene expression, and also for understanding the various mechanisms related to gene regulations. Recent advancements in cellular imaging and labeling have unfolded the ways scientist can look at RNA in individual cells. A variety of reliable and convenient reagents are now commercially available to label RNA in situ hybridization process of fixed cells. Techniques to study RNA transcripts in living cells are although considered more challenging, but are continually emerging and expanding. Multiple types of probe and labeling reagents for live-cell imaging are now commercially available. Genetically encoded probe based system has evolved as an important tool for monitoring RNA activities as they bypass the various limitations associated with the use of fluorescent probes. On the other hand, the high potential of aptamer-dye systems to image RNAs in various biological systems has been highlighted by several researchers. Overall, the field of RNA imaging is no longer in its infancy, and the scientific community is continuously exploring the technology to understand other important areas such as studying RNA dynamics in human diseases so that this knowledge could be used in designing newer therapeutic solutions.
Pankaj Kumar has written this article in his private capacity. No official support or endorsement by Indovax Pvt. Ltd. is intended or should be inferred. Dr. Kathryn McLaughlin added the slide-seq section and edited the FISH section in September 2019.
- Kwon S. Single-molecule fluorescence in situ hybridization: quantitative imaging of single RNA molecules. BMB Rep. 2013;46:65-72 pubmed
- Gall J, Pardue M. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969;63:378-83 pubmed
- Levsky J, Singer R. Fluorescence in situ hybridization: past, present and future. J Cell Sci. 2003;116:2833-8 pubmed
- Femino A, Fay F, Fogarty K, Singer R. Visualization of single RNA transcripts in situ. Science. 1998;280:585-90 pubmed
- Yao Q, Sun J, Ma H, Zhang A, Lin S, Zhu C, et al. Monitoring microRNAs using a molecular beacon in CD133+/ CD338+ human lung adenocarcinoma-initiating A549 cells. Asian Pac J Cancer Prev. 2014;15:161-6 pubmed
- Tyagi S, Kramer F. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303-8 pubmed
- Didenko V. DNA probes using fluorescence resonance energy transfer (FRET): designs and applications. Biotechniques. 2001;31:1106-16, 1118, 1120-1 pubmed
- Bertrand E, Chartrand P, Schaefer M, Shenoy S, Singer R, Long R. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437-45 pubmed
- Golding I, Cox E. RNA dynamics in live Escherichia coli cells. Proc Natl Acad Sci U S A. 2004;101:11310-5 pubmed
- Chubb J, Trcek T, Shenoy S, Singer R. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16:1018-25 pubmed
- Forrest K, Gavis E. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159-68 pubmed
- Zhang F, Simon A. A novel procedure for the localization of viral RNAs in protoplasts and whole plants. Plant J. 2003;35:665-73 pubmed
- Rook M, Lu M, Kosik K. CaMKIIalpha 3' untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci. 2000;20:6385-93 pubmed
- Shav Tal Y, Darzacq X, Shenoy S, Fusco D, Janicki S, Spector D, et al. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797-800 pubmed
- Janicki S, Tsukamoto T, Salghetti S, Tansey W, Sachidanandam R, Prasanth K, et al. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116:683-98 pubmed
- Daigle N, Ellenberg J. LambdaN-GFP: an RNA reporter system for live-cell imaging. Nat Methods. 2007;4:633-6 pubmed
- Lim F, Peabody D. RNA recognition site of PP7 coat protein. Nucleic Acids Res. 2002;30:4138-44 pubmed
- Valencia Burton M, McCullough R, Cantor C, Broude N. RNA visualization in live bacterial cells using fluorescent protein complementation. Nat Methods. 2007;4:421-7 pubmed
- Oguro A, Ohtsu T, Svitkin Y, Sonenberg N, Nakamura Y. RNA aptamers to initiation factor 4A helicase hinder cap-dependent translation by blocking ATP hydrolysis. RNA. 2003;9:394-407 pubmed
- Baskerville S, Zapp M, Ellington A. Anti-Rex aptamers as mimics of the Rex-binding element. J Virol. 1999;73:4962-71 pubmed
- Brodsky A, Silver P. Pre-mRNA processing factors are required for nuclear export. RNA. 2000;6:1737-49 pubmed
- Takizawa P, Vale R. The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc Natl Acad Sci U S A. 2000;97:5273-8 pubmed
- Ozawa T, Natori Y, Sato M, Umezawa Y. Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat Methods. 2007;4:413-9 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Aptamers and Affimers
- Biological Condensates
- Cloning and Expression Vectors, and cDNA and microRNA Clones Companies
- Live Cell Imaging
- LncRNA Research Resources
- MicroRNA Experimental Protocols
- MicroRNA Research Reagents
- MicroRNA Research Web Resources
- Optical Clearing of Biological Tissue
- RNA Extraction
- RNA Modifications
- RNA-seq
- Single Cell Technologies
- siRNAs and shRNAs: Tools for Protein Knockdown by Gene Silencing

