A comprehensive review of proteases and protease inhibitors commonly used in laboratory experiments, including survey results based on formal publications citing the applications of protease inhibitors or protease inhibitor cocktails.
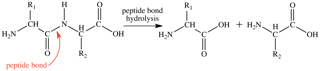
Proteins are chains of individual amino acids that are linked by covalent bonds — called peptide bonds — during the process of protein translation. New proteins are constantly being translated and old proteins degraded in the cell to ensure precise concentrations of the necessary functional protein to complete various cellular processes. The process of protein degradation, referred to as proteolysis, breaks proteins back down into individual amino acids. Proteolysis is accomplished by proteases, a group of enzymes whose mechanisms depend on the particular class of protease.
Proteases play many vital roles in cellular processes, including blood coagulation, food digestion, apoptosis, and autophagy. These processes are essential and, thus, proteases must work efficiently to ensure the survival of organisms. There are many different types of proteases in every cell of every organism, and these enzymes function in various regions of the cell, with different specificities and mechanisms of action.
Protease activity is highly regulated in the cell, and this regulation occurs through a variety of mechanisms. One example of regulated protease activation is during the initiation of apoptosis, or programmed cell death. Caspase activation and subsequent cleavage of downstream proteins play a significant role in apoptosis. Most cellular caspases exist as a procaspase (an inactive version of the protease) that must be proteolytically cleaved to exhibit protease activity. Proteins in the IAP (inhibitor of apoptosis) family block apoptosis by either preventing the cleavage of a procaspase [4, 5] or by directly inhibiting caspase activity [4, 6, 7].
The sub-cellular localization of proteases can also control their activity. Some proteases are sequestered within specific organelles such that they can only degrade proteins that are targeted to those organelles. For example, cathepsins are proteases predominantly localized to lysosomes. These proteases degrade proteins that are targeted to lysosomes [8]. In intact cells, proteins not localized to lysosomes do not come into contact with the cathepsins and , thus, are not proteolyzed by them.
When cells are lysed, regulatory mechanisms are disrupted and organelles can be ruptured. Cellular mechanisms of protease inhibition or protease sequestration will thus be eliminated. Normally sequestered proteases can then act on proteins with which they don’t generally come into contact. Previously inactive proteases might become activated and begin cleaving a variety of proteins. Because of the vast number and types of proteases present in organisms commonly used for protein expression (Table 1), it is easy to imagine that proteins of interest can be subject to extensive proteolysis upon cell lysis.
| Organism | Number of proteases |
|---|---|
| Escherichia coli K-12 | 1436 |
| Saccharomyces cerevisiae | 216 |
| Pichia pastoris | 125 |
| Homo sapiens | 1121 |
Because of this potential unwanted protease activity, it is necessary to take measures to prevent proteolysis during protein isolation protocols. Generally, only then can full-length, functional protein be isolated for use in experimental studies. Protease inhibitors are typically used in these types of studies, and the type of inhibitor(s) to use depends on the types of proteases that need inhibiting.
Proteases can be classified by their mechanism of action and their substrate specificity.
Broadly, proteases can be characterized as endopeptidases or exopeptidases. Endopeptidases cleave bonds within the protein and are usually very specific for particular amino acid sequences. Exopeptidases cleave a single amino acid from the terminus of a protein, and are less specific toward the amino acid that is being cleaved. Exopeptidases are themselves classified as those that cleave only from the C-terminus (carboxypeptidases), only from the N-terminus (aminopeptidases), or both termini (dipeptidases). Both endopeptidases and exopeptidases can be very problematic during protein purification. Endopeptidases can recognize regions in the protein of interest and perform proteolysis to digest the protein into many smaller pieces that are useless in experimental studies. Exopeptidases can remove just a few amino acids which may dramatically affect protein function but is often not detectable by conventional methods.
Proteases are also characterized by their mechanism of action; that is, the amino acids that are involved in the catalytic site of the enzyme. The catalytic site contains the amino acids that directly play a role in facilitating peptide bond hydrolysis (Figure 1). This process requires a nucleophile to initiate the reaction, which can either be an active site amino acid side chain or a water molecule. During the later stages of proteolysis, the two parts of the hydrolyzed protein are released and the protease active site returns to its initial state, ready to bind another substrate for proteolysis.
Proteases are typically grouped into four major classes based on their active site residues: serine proteases, cysteine (thiol) proteases, aspartic proteases, and metalloproteases. Serine and cysteine (thiol) proteases have an amino acid within the active site that performs the initial nucleophilic attack, while aspartic proteases and metalloproteases activate a water molecule to perform the initial nucleophilic attack.

- Serine proteases (Figure 2) act through a catalytic triad composed of a serine, a histidine residue, and an aspartate residue.
- Cysteine (thiol) proteases contain a catalytic dyad with a histidine residue and a cysteine residue. The cysteine performs the nucleophilic attack to initiate peptide bond hydrolysis.
- Aspartic proteases (Figure 3) contain a catalytic dyad with two aspartate residues.
- Metalloproteases require a zinc ion (or, more rarely, a different divalent metal ion) that is coordinated to the protein by three amino acids, whose identity can vary. The metal ion activates the water molecule, such that it can perform a nucleophilic attack on the carbonyl carbon to break the peptide bond.
Endoproteases specifically recognize certain amino acids or types of amino acids. The amino acids that form the peptide bond, as well as neighboring residues, may also play a role in specificity of the protease. This recognition is mediated by a protease’s specificity pocket, a region within the protease around the active site that binds some amino acid side chains more favorably than others. Some of the most common protease include the following:

- Trypsin-like proteases predominantly cleave proteins at the carboxyl side of arginine or lysine (except when that residue follows a proline).
- Chymotrypsin-like proteases preferentially cleave on the carboxyl side of large aromatic residues (tryptophan, tyrosine, or phenylalanine).
- Caspase-like proteases predominantly cleave on the carboxyl side of aspartate, but one caspase in Drosophila has been shown to also cleave on the carboxyl side of glutamate [10].
- Elastase-like proteases predominantly cleave on the carboxyl side of small, aliphatic amino acids (glycine, alanine, or valine).
Protease inhibitors are molecules that block the activity of proteases, and typically function on classes of proteases with similar mechanisms of action. Protease inhibitors can either be in the form of proteins, peptides, or small molecules (Figure 4). Naturally occurring protease inhibitors are usually proteins or peptides. Protease inhibitors used in experimental studies or drug development are often synthetic peptide-like or small molecules. Many different protease inhibitors are commercially available to use experimentally in both in vitro and in vivo assays and during protein purification.
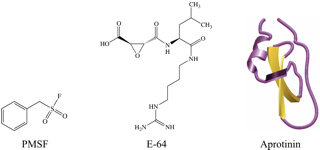
Protease inhibitors can work in many different ways to inhibit the action of proteases. These inhibitors can be classified by the type of proteases they inhibit and the mechanism by which they inhibit those enzyme. While commercial protease inhibitors are typically sold based on the class of protease they inhibit, understanding the various mechanisms by which inhibitors function is essential for a comprehensive understanding of inhibition and for developing protease inhibitors as a therapeutic strategy.
Reversible inhibitors usually bind to the protease with multiple non-covalent interactions, without any change to the inhibitor itself. These inhibitors can be removed by dilution or dialysis. Reversible inhibitors include competitive inhibitors, uncompetitive inhibitors, and non-competitive inhibitors.
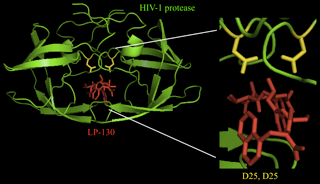
- A competitive inhibitor binds to the active site of the protease, competing with substrates for access to the active site residues. One example of a competitive inhibitor is aprotinin, which inhibits many serine proteases [11]. Competitive inhibitors are often similar in structure to the transition state of natural substrates. The transition state of the substrate is an intermediate state of the structure that typically binds most tightly with the enzyme. Therefore, compounds mimicking this structure bind to the enzyme with greater strength than the substrate (in its initial state) can, and thus the normal enzymatic reaction cannot proceed. An example of this type of transition state analog is LP-130 (Figure 5), an HIV-1 protease inhibitor.
- Uncompetitive inhibitors bind to the protease only when it is already attached to a substrate. Uncompetitive inhibitors have been identified for HIV-1 protease [12] and the NS2B-NS3 proteinase of the West Nile virus [13].
- Non-competitive inhibitors bind to the protease with similar affinities, regardless of the presence of a bound substrate. These molecules inhibit protease activity through an allosteric mechanism. BBI, a trypsin inhibitor from soybeans [14] and aminoglycosides, inhibitors of the anthrax lethal factor protease [15] are examples of non-competitive inhibitors.
Irreversible inhibitors function by specifically altering the active site of its specific target protease, often through the covalent bond formation. They can be more appropriately called inactivators. Upon binding to the inhibitor, a protease’s active site is altered, and it can no longer perform peptide bond hydrolysis. Some of such inhibitors do not actually covalently bind to the protease, but interact with such a high affinity, that they are not easily removed. Whether the inhibition is reversible or not has implications in using an inhibitor: for a reversible inhibitor, the concentration of the inhibitor must be maintained throughout a protocol, such as during protein purification, while for an irreversible inhibitor, once all proteases have been inactivated, there is no need to maintain the inhibitor concentration.
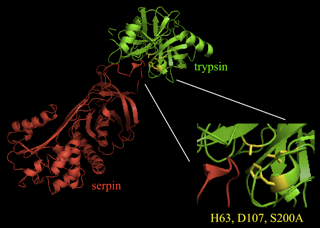
Suicide inhibitors, typically analogs of the substrate, are irreversible inhibitors that covalently bind to proteases. An example of a suicide protease inhibitor is the serpin family of proteins, which play a role in blood coagulation and inflammation. Figure 6 shows a crystal structure of a serine protease (trypsin) interacting with a serpin. A loop in serpin serves as a substrate analog. The serine residue in the active site of trypsin forms a nucleophilic attack on a carbonyl carbon of the substrate analog, inducing a conformational change in the enzyme that renders the remainder of the peptide bond hydrolysis reaction unfavorable. Thus, the serpin remains covalently bound to the protease so that the enzyme is no longer available for binding to substrates.
Protease inhibitors can be purchased individually or as a concentrated cocktail containing multiple protease inhibitors in appropriate concentrations. Individual protease inhibitors are ideal for proteolytic assays of already purified proteins. Often times, enzymatic assays require a control to show that the enzyme of interest is performing the monitored action; thus, adding a specific protease inhibitor can adequately serve as a control. Purchasing individual protease inhibitors may also be useful when the protein being purified is a protease itself. In this case, the presence of functional proteins and the purity is often assessed by enzymatic assays. For this type of purification, multiple protease inhibitors can be added, excluding those that inhibit the protease of interest. Some of the most common protease inhibitors that are used during protein purification are listed in Table 2. Most offer broad inhibition of one or more classes of proteases.
| Inhibits | Reversibility | Advantages/Disadvantages | |
|---|---|---|---|
| AEBSF / Pefabloc | serine proteases | irreversible | Can covalently modify some amino acid residues with a 183 Da AEBS group, leading to problems with mass spectrometry and gel electrophoresis Very stable in aqueous solutions, compared to most other serine protease inhibitors Very low toxicity and can be used in cell culture or animals in vivo. |
| Aprotinin | serine proteases | reversible | Can stick to some dialysis tubing and column matrices with low ionic strength Can be fluorescently labeled without affecting its inhibitory activity Dissociates from protease at pH<3 or pH>10 |
| Bestatin | some aminopeptidases | reversible | Low stability in aqueous solutions |
| E-64 | cysteine proteases | irreversible | Can be coupled to a matrix for affinity purification of cysteine proteases [16] Very soluble in aqueous solutions High specificity (does not affect other cysteine residues) Cell permeable and low toxicity makes it useful for in vivo studies |
| EDTA/EGTA | metalloproteases | reversible | Not compatible with immobilized metal affinity chromatography (IMAC) Not compatible with two-dimensional gel electrophoresis Very soluble and stable in aqueous solutions |
| GM 6001 | matrix metalloproteinases | reversible | Can be injected in vivo, e.g., injection i.p. to mice at 100 µg/g daily [17] |
| Leupeptin | serine/cysteine proteases | reversible | Low stability at the working concentration Not cell permeable May affect assays for determining protein concentration |
| Pepstatin | aspartic proteases | reversible | Insoluble in aqueous solutions High stability |
| PMSF | serine proteases | irreversible | Is a neurotoxin and should be handled carefully Limited solubility in aqueous solutions Very unstable in aqueous solutions |
Protease inhibitor cocktails are often used for their reliability and reproducibility. Cocktails contain multiple protease inhibitors in the appropriate relative amounts, eliminating the need for trial and error in determining the required types and amounts of inhibitors to use. For example, MilliporeSigma Protease Inhibitor Cocktail P2714 contains AEBSF, aprotinin, bestatin, E-64, EDTA and Leupeptin. They also reduce the opportunities for human or pipetting error, by requiring only one solution versus multiple different solutions. These cocktails come in both liquid and solid forms.
Protease inhibitors, both individual and cocktails, are available from a number of commercial sources (Table 3). MilliporeSigma is one of the largest providers of inhibitor cocktails and individual protease inhibitors. MilliporeSigma cOmplete tablets (Figure 7) are among the most commonly used protease inhibitor cocktails. These tablets are added to a specific volume of the buffer to inhibit the most abundant proteases. MilliporeSigma cOmplete tablets have been shown to successfully inhibit a broad range of proteases in lysates from many organisms, including E. coli, yeast, insects, and mammals. These tablets are available both with and without EDTA. Figure 8 shows SDS-PAGE results of protein purified with and without the addition of a MilliporeSigma cOmplete ULTRA (EDTA-free) protease inhibitor cocktail tablet. The yield of full-length protein in the absence of protease inhibitors is less than half of that obtained when the protease inhibitor cocktail is used.

Protease inhibitors in tablet form are added to an appropriate volume of the buffer, and the tablets dissolve to bring the concentration of each protease inhibitor to the appropriate level. Adding protease inhibitors in tablet form is very convenient as no pipetting is involved. In cases where tiny volumes of the buffer are required, protease inhibitor cocktails in liquid form might be more suitable to avoid wasting excess buffer or inhibitors, as the tablets require the preparation of a set volume.

EMD Millipore (now part of MilliporeSigma) sells various Calbiochem protease inhibitor cocktails for different purposes. The components of these cocktails are given on their website and can help consumers choose which product is best for their application. Cocktails that inhibit multiple classes of proteases (e.g., Protease Inhibitor Cocktail Sets I-VIII) and cocktails that are developed for inhibiting a broad range of proteases from the same class (e.g., Serine Protease Inhibitor Cocktail Set I) are available. These are typically supplied in DMSO or as a lyophilized powder that can be reconstituted to yield a concentrated stock solution.
Thermo Scientific sells protease inhibitor cocktails in both liquid and tablet form. Halt Protease Inhibitor Cocktail is a 100X solution that is stable at 4°C. It contains a combination of AEBSF, aprotinin, bestatin, E-64, leupeptin, and pepstatin A dissolved in DMSO. A vial of EDTA is provided for the option of metalloprotease inhibition. The same combination of protease inhibitors is sold in tablet form, both with and without EDTA.
G-Biosciences sells FOCUS™ ProteaseArrest™, a protease inhibitor cocktail that is compatible with mass spectrometry and two-dimensional gel electrophoresis. This cocktail contains protease inhibitors exhibiting broad inhibition and includes an alternative to EDTA for inhibiting metalloproteases. Recom ProteaseArrest™ is a cocktail specific for the purification of histidine-tagged proteins expressed in bacteria. Other ProteaseArrest™ formulations are available from G-Biosciences for proteins isolated from different types of organisms, and these formulations contain varying amounts of inhibitors depending on the relative quantity of various proteases in the organisms. Similarly, MilliporeSigma sells protease inhibitor cocktails reportedly optimized for mammalian tissue/cell extracts, plant tissue/cell extracts, fungal/yeast cell extracts and mammalian secreted proteins.
Many other companies also sell protease inhibitors individually, as cocktails, and as sets of multiple individual inhibitors. These sets are useful for determining which protease inhibitors are required for a certain application. They can also be useful when a specific protease inhibitor included in most commercial cocktails is known to interfere with the protein of interest or downstream application. Sigma Aldrich, GE Healthcare, Promega, Cell Signaling Technology, Clontech, and Santa Cruz Biotechnology, Inc. all sell protease inhibitors in various formats.
Selective suppression of microbial proteases can be achieved by application of monoclonal antibodies. A novel approach for the generation of protease inhibitory antibodies by the selective conversion has recently been demonstrated [18]. The authors of the study used a dual-color flow cytometry to perform the selection on matrix metalloproteinase (MMP)-9 and the counter-selection on MMP-14 catalytic domains. The results have shown successful conversion of MMP-14 inhibitory antibodies to MMP-9 inhibitory antibodies with high selectivity.
Another study has described a functional selection of protease inhibitory antibodies, which has been based on co-expression of recombinant proteins in Escherichia coli, a protease of interest and a β-lactamase with a protease cleavable peptide sequence [19]. Several antibodies suppressing different proteases, such as matrix metalloproteinases, beta-secretase 1 and cathepsin B, have been obtained. The protease inhibitory antibodies have been effective in both cellular assays and animal models.
A recent study has described the isolation and characterization of PPF-BBI, a novel protease inhibitor obtained from the skin secretion of the Fukien gold-striped pond frog, Pelophlax plancyi fukienesis [20]. The compound showed antimicrobial activity against C aureus, C albicans and E coli.
Another study has presented GP205, a new inhibitor of NS3/4Aserine protease with low nanomolar activities against several hepatitis C virus (HCV) replicons. The analysis of GP205 pharmacokinetics demonstrated prolonged plasma half-life. This serine protease inhibitor might be valuable for the development of new HCV treatments [21].
A flavonoid library has recently been screened to identify compounds able to suppress MERS-CoV 3C-like protease (3CLpro) produced by Middle East respiratory syndrome-coronavirus [22]. Flavonoids herbacetin, isobavachalcone, quercetin 3-β-d-glucoside and helichrysetin have been shown to decrease the activity of 3CLpro and can therefore be applied to the development of new treatment approaches against coronavirus.
Labome manually surveys formal publications for the reagents used. Table 3 lists the commonly cited protease inhibitors. Protease inhibitor cocktails are very commonly used. Almost half of the publications surveyed by Labome cited MilliporeSigma protease inhibitor cocktail. Litke JL et al, for example, included Halt protease and phosphatase inhibitor cocktail from Thermo Fisher (78440) in the RIPA buffer to lyzing Hela cells for Western blot [23].
| Inhibitor | Supplier | Catalog Numbers and Sample References |
|---|---|---|
| AEBSF | MilliporeSigma | [24] |
| aprotinin | MilliporeSigma | [25] |
| EDTA | MilliporeSigma | [25] |
| STEMCELL Technologies | [26] | |
| EGTA | MilliporeSigma | [27, 28] |
| leupeptin | MilliporeSigma | [29] |
| pepstatin | MilliporeSigma | [24, 30] |
| PMSF | MilliporeSigma | [31, 32] |
| Carl Roth | [33] | |
| protease and phosphatase inhibitor cocktails | MilliporeSigma | [30, 34] |
| Thermo Fisher | [35], 78430 [36, 37], 78443 [38], 78429 [39], 78440 [23, 40] | |
| Cell Signaling Technology | [41] | |
| protease inhibitor cocktail | MilliporeSigma (including Roche) | [42, 43] |
| valine-pyrrolidide | MilliporeSigma | [25] |
There are many things to take into consideration when choosing the appropriate protease inhibitor(s) for a particular process. The most important factor is the purpose of protease inhibition—whether inhibitors are needed to prevent proteolysis during protein purification, to inhibit a purified enzyme as an experimental control, or for use in living organisms to affect physiological processes. The following section includes some major questions and concerns to consider when using protease inhibitors.
The application plays a significant role in the type of protease inhibitors that should be used. For in vitro and in vivo assays, the choice of protease inhibitor depends on the particular enzyme or physiological process being studied. For enzymatic assays, the inhibitor should not interfere with the method of detection. Although if inhibition is reversible, and another detection method (e.g., ELISA) is available, it may not matter if the target protein is inhibited during purification provided that the inhibitor is absent from the final preparation. It should be noted that some protease inhibitors are not specific to proteases. For example, PMSF irreversibly inhibits many, but not all, members of the serine hydrolase family. This family includes proteases, such as trypsin and chymotrypsin, but also includes a great many other enzymes that hydrolyze non-protein substrates (e.g., acetyl-cholinesterases, acyl-CoA hydrolases and lipases) [44-46]. For studies in living organisms, the inhibitors must be cell permeable, highly specific, and non-toxic. For the broad inhibition of proteases to assist in purifying a full-length, functional protein, multiple proteases are typically required.
Many protease inhibitors are not stable for long periods, either as a stock solution or at their working concentration. It is critical to prepare solutions according to vendor instructions, as some protease inhibitors are more stable under certain conditions than others. Typically, if stock solutions have been stored as suggested and working solutions prepared immediately before use, inhibitors should retain sufficient function. However, some protease inhibitors, such as PMSF, are very unstable and may need to be added multiple times during lysis and purification to ensure protease inhibition.
Several companies sell reagents as a measure to check for protease function, and these can be used to confirm adequate inhibition. MilliporeSigma sells a Universal Protease Substrate, which utilizes an absorbance-based assay to monitor degradation of resorufin-labeled casein. This substrate can be used if there is evidence to suspect inadequate protease inhibition. Other companies, such as Calbiochem (EMD Millipore), sell similar reagents. Typically, it is a better use of time and energy to make a fresh protease inhibitor solution if it is suspected that proteases have not been well inhibited. However, if proteolysis is a recurring problem, these commercial substrates can help in determining more appropriate conditions for protease inhibition.
Protease inhibitors should be added to the lysis buffer so that inhibition begins immediately upon cell lysis, when the proteases are released from their cellular compartments or their regulation is otherwise disrupted. These protease inhibitors should remain in the buffers used in the purification scheme until most of the contaminating proteases have been sufficiently separated from the protein of interest. Typically, if the first chromatographic step is stringent (e.g., affinity chromatography), protease inhibitors only need to be present through the wash step of this chromatographic procedure. The elution buffer and remaining purification buffers do not usually require protease inhibitors. This is a significant financial consideration for large-scale protein purification efforts where large quantities of buffers may be necessary; the inclusion of high concentrations of protease inhibitors throughout large-scale purifications can be prohibitively expensive.
However, many proteases are very efficient catalysts with high turnover numbers and so if even a small amount of a protease co-purifies with the target protein it can cause problems. This can result in clear degradation of the target protein (see Figure 8) or it may not be evident until N-terminal or mass spectroscopic analysis reveals that the purified protein preparation contains a variety of species with different N-termini. For many applications, such micro-heterogeneity may not be an issue, but for others it may cause significant problems. For example, in a structural biology context, such N-terminal heterogeneity may affect the ability to crystallize a protein and/or the quality of any crystals obtained [47-49]. Thus, in some cases, it may be necessary to include inhibitors against one or more classes of protease into the later stages of the purification protocol. The use of protease assays (as discussed above) can be used to identify the protease class(es) that needs to be blocked.
Cell lysis and purification should be performed at a low temperature. Typically, lysis and purification are performed on ice or at 4°C. This not only helps with protein folding and stability but also slows down the rate of proteolysis by contaminating proteases. Additionally, the faster the contaminating proteases are removed from the protein of interest, the less time they have to interact with and possibly degrade the proteins of interest. Do not let cell lysates sit at intermediate steps for long periods, even on ice. Proceed through the purification steps as quickly as possible to remove as many proteases as possible, and store purified protein appropriately.
If proteolysis of a recombinant protein is a particular problem, several approaches can be considered. For E.coli expression it is possible that lowering the growth temperature will reduce proteolysis of the target protein that occurs before cell harvest and lysis [50]. It may also be possible to reduce intracellular proteolysis of the target protein by directing expression to the periplasm and thus reducing exposure to intracellular proteases [50, 51]. Similarly, to avoid proteolysis by mammalian intracellular proteases, it may be possible to use an expression system that drives secretion of the recombinant protein into the culture medium [52]. Considering the different range of proteases produced by different organisms, it may also be possible to reduce, or even eliminate, proteolysis issues by switching expression to a different host organism (e.g., E. coli to baculovirus/insect cells).
The best place to start in choosing the correct combination of protease inhibitors for use during cell lysis and protein purification is to try a commonly used protease inhibitor cocktail. For the majority of lysates and applications, these are sufficient. However, if a full-length protein is not obtained or problems are experienced in downstream applications, specific protease inhibitors or protease inhibitor sets can be used to determine the most appropriate combination of protease inhibitors.
Protease inhibitors have been tested in pre-clinical and clinical trials. Suppressors of convertase subtilisin-kexin type 9 were shown to decrease the level of low-density lipoproteins and have been approved for the treatment of patients with severe hyperlipidemia [53]. Dipeptidyl peptidase-4 (DPP-4) suppressors effectively lower glucose levels and are used for treating patients with type 2 diabetes [54]. DPP-4 inhibitor linagliptin was demonstrated to suppress the development of diabetic retinopathy in an experimental model [55]. A recombinant serine protease inhibitor (rBmTI-A) has been evaluated in a mouse model of allergic lung inflammation [56]. The proteolytic activity, polymorphonuclear and eosinophilic responses and proinflammatory cytokine production have been decreased by the treatment with rBmTI-A in Balb/c mouse lungs, suggesting potential application of this protease inhibitor for asthma therapy. Also, the effects of protease inhibitor MG-132 on sepsis-induced lung injury have been studied using a Sprague Dawley rat model [57]. The authors have found that MG-132 protects against acute lung injury through inhibition of mTOR/4EBP1/EIF4E pathway. In addition, the effects of secretory leukocyte protease inhibitor (SLPI) on squamous cell carcinoma (SCC) have recently been evaluated [58]. The study showed that SLPI suppressed human papilloma virus-mediated phenotypes in SCC cells via an upregulated NF-κB signaling pathway. Inhibitors of cysteine protease cathepsin K increase bone mineral density in individuals with postmenopausal osteoporosis [59]. Cathepsin K suppressor odanacatib was shown to decrease bone resorption in both male and female patients with osteoporosis [60].
- Daniel Kahne, W. Clark Still (1988). Hydrolysis of a peptide bond in neutral water. Journal of the American Chemical Society 1988 110 (22), 7529-7534. Available from: dx.doi.org/10.1021/ja00230a041
- Martin, R. B. (1998), Free energies and equilibria of peptide bond hydrolysis and formation. Biopolymers, 45:351–353. Available from: dx.doi.org/10.1002/(SICI)1097-0282(19980415)45:5<351::AID-BIP3>3.0.CO;2-K
- Aprotinin. Available from: www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/aprotinin-monograph.html
- Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17:931-9 pubmed
- LaCount D, Hanson S, Schneider C, Friesen P. Caspase inhibitor P35 and inhibitor of apoptosis Op-IAP block in vivo proteolytic activation of an effector caspase at different steps. J Biol Chem. 2000;275:15657-64 pubmed
- Datta R, Oki E, Endo K, Biedermann V, Ren J, Kufe D. XIAP regulates DNA damage-induced apoptosis downstream of caspase-9 cleavage. J Biol Chem. 2000;275:31733-8 pubmed
- Hawkins C, Yoo S, Peterson E, Wang S, Vernooy S, Hay B. The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J Biol Chem. 2000;275:27084-93 pubmed
- Chabbat J, Porte P, Tellier M, Steinbuch M. Aprotinin is a competitive inhibitor of the factor VIIa-tissue factor complex. Thromb Res. 1993;71:205-15 pubmed
- Sperka T, Pitlik J, Bagossi P, Tozser J. Beta-lactam compounds as apparently uncompetitive inhibitors of HIV-1 protease. Bioorg Med Chem Lett. 2005;15:3086-90 pubmed
- Kuzmic P, Cregar L, Millis S, Goldman M. Mixed-type noncompetitive inhibition of anthrax lethal factor protease by aminoglycosides. FEBS J. 2006;273:3054-62 pubmed
- Govrin E, Levine A. Purification of active cysteine proteases by affinity chromatography with attached E-64 inhibitor. Protein Expr Purif. 1999;15:247-50 pubmed
- Kraut D, Goff H, Pai R, Hosea N, Silman I, Sussman J, et al. Inactivation studies of acetylcholinesterase with phenylmethylsulfonyl fluoride. Mol Pharmacol. 2000;57:1243-8 pubmed
- Saario S, Savinainen J, Laitinen J, Jarvinen T, Niemi R. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem Pharmacol. 2004;67:1381-7 pubmed
- EMBL Protein Expression and Purification Core Facility. Available from: www.embl.de/pepcore/pepcore_services/index.html
- Scott M, Modha S, Rhodes A, Broadway N, Hardwicke P, Zhao H, et al. Efficient expression of secreted proteases via recombinant BacMam virus. Protein Expr Purif. 2007;52:104-16 pubmed
- Inzucchi S, Bergenstal R, Buse J, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-9 pubmed publisher
- Nakamura T, Shiraki M, Fukunaga M, Tomomitsu T, Santora A, Tsai R, et al. Effect of the cathepsin K inhibitor odanacatib administered once weekly on bone mineral density in Japanese patients with osteoporosis--a double-blind, randomized, dose-finding study. Osteoporos Int. 2014;25:367-76 pubmed publisher
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
