A comprehensive review of phage-display technology for the production of recombinant monoclonal antibodies.
Monoclonal antibodies have contributed significantly in various fields, such as molecular biology, pharmaceutical, and medical research, and also aided in the treatment of diseases such as cancer and infectious diseases [1-3]. Recombinant antibodies (rAbs) have emerged as a valuable and practical means for research, diagnosis of various diseases, and as one of the fastest growing class of therapeutic proteins. A variety of systems for rapid and large-scale production of recombinant antibodies have been employed to meet the increasing demand for the antibodies. The benefit of huge amount and consistent quality of the in vitro antibody production methods have interested researchers to make improvements and try various production systems, viz. Gram-negative and Gram-positive bacteria, yeast (for example Adimab platform [4] ) and filamentous fungi, insect / mammalian cell lines, or more recently in transgenic plants and animals [5-7] for economic and large-scale production of rAbs. The other advantages offered by the rAbs include improvements in intrinsic properties such as immunogenicity, affinity, specificity, and stability of antibodies by a variety of mutagenesis techniques [8-10]. Libraries of antibody gene fragments can be cloned into expression vectors and displayed as a single-chain variable fragment or Fab-fragment antibodies on the surface of filamentous bacteriophage [11]. This technique, known as phage display, has helped enormously to study molecular biology mechanisms involving protein-protein [12] or protein-nonprotein interactions [13]. There are many excellent and comprehensive reviews on this topic, and one of the most recent ones is by the Bio-Rad group [14]. Phage display with pre-selected antibodies has also been exploited to enable barcoding of antibodies and thus highly multiplexed and quantitative cell-surface protein profiling [15]. Phage display can also be used to identify other types of protein affinity reagents such as Ras-binding domains [16].
The technique was initially demonstrated by George P. Smith in 1985 [17]. McCafferty and colleagues, in 1990, confirmed its use for the production of recombinant antibodies with desired specificities [18]. Since then, many research groups have contributed towards essential improvements and modifications of the technique for its potential applications in basic and applied biological sciences. Phage display technology allows the isolation of human antibodies against almost any antigen through the clonal selection of antibody fragments in a prokaryotic system, thus facilitating both immunotherapy and in vivo diagnosis. The range of antibodies from antibody libraries is limited by the initial calculated complexity of the antibody fragment gene library. Because the antigen-binding fragment is a heterodimer, the library complexity is increased by the random combination of heavy- and light-chain sub-libraries.
As previously mentioned, phage display technology refers to the use of phages for display of random peptides, foreign proteins or protein domains, as fusion proteins, on their surface [19]. Phages are viruses which infect bacterial cells. Mostly, the vectors used in recombinant DNA technology, are bacteriophages which infect Escherichia coli, the standard recombinant DNA host. An essential feature of such recombinant DNA vectors is that they can contain stretches of foreign DNA, which gets expressed in the host cell when the vector replicates. A phagemid vector, termed so for its phage origin genome, provides such features and enables expression of the foreign DNA, purposely introduced in the vector in such a way that it is expressed in conjunction with a phage protein, as a fusion protein, for display on the phage surface [19]. A variety of bacteriophages, viz. Ff filamentous phage, Lambda and T7 [20, 21] have been utilized in phage display with the most common being the Ff phage family (e.g., M13, fd-phage). These have been preferred, amounting to their potential as excellent cloning vehicles and simplicity for correct assembly of longer phage particles [21]. The PIII or PVIII surface proteins of the phage are used for display of recombinant peptides. Several options aid in avoiding the limitations of the conventional display system [22]. M13 filamentous phage-based libraries like Ph.D.TM-7, Ph.D.TM-12, Ph.D.TMC7C from New England Biolabs, or spherical T7 phage, T7Select® from Merck are available.
The most productive phage in nature is the filamentous phage which infects Escherichia coli. It generates titers of up to 1013 per ml of bacterial culture. These bacteriophages infect a range of Gram-negative bacteria using the bacterial pili as a receptor. The best characterized of these phages (M13, fl, and fd) are collectively referred to as the Ff phage. These infect E.coli containing the F conjugative plasmid. The first phage display libraries of peptides and antibodies were published in 1990-1991 [23-26], after which the technique underwent numerous improvements for its varied applications.
The vectors designed by combining portions of plasmid and phage genome, for cloning and expression of fusion proteins are known as phagemid vectors. These vectors enclose an M13 phage origin of replication and packaging signal along with an origin of replication and the expression system of the plasmid. Usually, the expression plasmids are composed of multiple cloning sites, an antibiotic resistance marker, epitope tags such as a hexahistidine tag or a c-myc tag [27], and a lacZ promoter [28]. The phagemids habitually contain a gene sequence which codes for coat protein which will be fused to the foreign DNA that is to be expressed [23, 29], and a stop codon (usually amber), to permit host specific expression of the pIII fusion protein or a soluble fusion partner [30]. Phagemids can uphold themselves as plasmids, allowing the expression of the desired protein in the bacterial cell, but they do not have the other genes necessary for phage assembly. Therefore, infection of the phagemid-transformed host cells, with a helper phage becomes essential for the production of viable phage particles. The helper phage is the bacteriophage itself, which is engineered to provide the proteins essential for phage assembly. The phage-foreign fusion proteins can be displayed as either N-terminal fusions with pIII, pVII, pVIII, or pIX, for large proteins [31, 32] or C-terminal fusions with pIII, pVI, or pVIII proteins of the phage [33, 34]. The phage particles can be used in binding selections, and the binding clones can be multiplied through infection of E. coli host.
A drawback of the phagemid system is the reduction in the number of recombinant fusion proteins displayed on each phage particle due to competition between the wild-type coat protein and a fusion coat protein for integration into the phage particle [11]. Because of this problem, a modified helper phage, known as the hyperphage, was designed [35] which reportedly enhanced the yields of recombinant phages displaying the recombinant antibodies by more than two orders of magnitude. Hyperphages have a wild-type pIII phenotype and are therefore able to infect F+ E. coli cells with high efficiency; however, they lack a functional pIII gene which renders these particles non-functional and thus require the phagemid-encoded pIII–antibody fusion for complete phage assembly (Figure 1). The outcome is a significant rise in the proportion of recombinant antibody displaying phages. It was reported that the antigen-binding activity also got affected by the use of the hyperphage. As a result, the hyperphage was found to be valuable in cases of selecting antibodies against rare epitopes.

Apart from this, specific mutations were also introduced in the coat proteins for the development of a hybrid phage display system [27], in which, two copies of the gene encoding coat protein exist: one with the scFv fusion and the other coding the natural non-fusion protein. Finally, the amount of recombinant proteins displayed is determined by more than a few factors, viz., type of coat protein chosen for fusion (pIII or pVIII), display system chosen for expression (phage or phagemid), and the choice of helper phage in case a phagemid system is used.
Some of the most widely used and commercially available phage display vector systems include, pSEX81 [36, 37], pCANTAB 5E [38-40], pCOMB3 [41] or its derivates, for example, pComb3XSS (Addgene 63890) [42, 43].
The phagemid vector, pSEX81, has been engineered for expression of functional single-chain Fv antibody-pIII fusion proteins on the surface of M13 bacteriophages. The phagemid was constructed explicitly for cloning of immunoglobulin heavy and light chain gene fragments isolated from human or mouse antibody libraries. The cassette vector, pSEX81, was designed for the convenient insertion of heavy and light chain variable domain coding regions and the production of a functional single chain of M13 bacteriophages. The corresponding DNA fragments of human or mouse origin can be amplified by PCR using the degenerate IgG/IgM, or IgG primer sets respectively, available from the same company. The amplified gene fragments encoding variable heavy or light domain are cloned in-frame between a signal peptide sequence of bacterial pectate lyase (pelB) for secretion of the fusion protein into periplasmic space and pIII gene of M13 bacteriophage.
The VH and VL genes are joined by a DNA fragment coding for a flexible 18 amino acid residue linker containing first six amino acids of CH1 constant region domain and the hydrophilic pig brain alpha tubulin peptide sequence "EEGEFSEAR". Vector backbone further provides a strong promoter, the T7 terminator, ColE1 origin of replication, intergenic region of phage F1 and an ampicillin resistance marker for selection. In pSEX81 the recognition sites of restriction endonucleases Nco I and Hind III allow insertion of VH gene fragments. For the insertion of a VL gene fragment, sites of restriction endonucleases Mlu I and Not I are present.
The systems for phage display can be categorized along the lines of the arrangement of the phage genes coding for coat proteins [44, 45]. A ‘type 3’ vector, consists of a single phage chromosome (genome) containing a single gene III for expression of foreign DNA and a single type of pIII protein molecule. In theory, the foreign peptide encoded by the insert gets displayed on all the five pIII molecules on a virion (though practically the host proteolytic enzymes remove the foreign peptide from some or even most of the pIII copies, especially if the foreign peptide is large). Likewise, the ‘type 8’ and ‘type 6’ vectors display foreign peptides on every copy of pVIII and pVI, respectively [46-48]. In a ‘type 88’ vector, the phage genome bears two VIII genes, which encodes for two different types of pVIII molecules, a recombinant one, bearing the foreign DNA and the wild-type one. This system allows the display of larger proteins on the phage surface. In the same way, the ‘type 33’ vector bears two genes III. A ‘type 8+8’ system is different in the manner that the two VIII genes are on separate genomes. The wild-type being on a phage (helper phage), while the recombinant one on the phagemid [28, 49]. The ‘type 3+3’ and the ‘type 6+6’ systems are reminiscent of ‘type 8+8’ systems.
Production of large quantities of antibodies becomes challenging due to the high complexity of the immunoglobulin molecules. The immunoglobulin G is a heterotetramer, consisting of two different polypeptide chains joined by the inter-chain disulfide bonds. The antibody light chain and heavy chain consist of several domains known as “immunoglobulin folds”, each of which requires intra-domains disulfide bond for stabilization. An oxidizing environment along with the complex apparatus is much needed for efficient and correct conformation of a large number of disulfide bonds together with folding and assembly of four chains to one IgG molecule. The in vitro hosts for the production or selection of antibodies, generally have less optimal conditions for expression of a correct and complete immunoglobulin molecule. Thus, many researchers opted for other smaller sized formats of antibodies, which retained the antigen-specificity and also provided significant advantages.
By far, the ‘Fv’ fragment is the smallest antigen-binding fragment of immunoglobulin retaining complete antigen binding site and consists of only the variable fragments of an antibody structure. A single-chain variable fragment (scFv) is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins (Figure 2), connected with a short linker peptide often to about 25 amino acids, which helps to improve the stability of the short antibody. The flexibility and solubility of the linker are due to the glycine and serine or threonine amino acid residues, respectively. The linker can either connect the N-terminus of the VH with the C-terminus of the VL or vice versa. This protein retains the specificity of the original immunoglobulin, despite the removal of the constant regions and the introduction of the linker. These molecules were created to facilitate phage display, where it is highly convenient to express the antigen-binding domain as a single peptide. As an alternative, scFv can be constructed directly from a sub-cloned heavy and light chains derived from a hybridoma. Since the scFvs are only half the size of a Fab fragment and still retain the original specificity of the parent immunoglobulin, they have significant applications in techniques, such as flow cytometry, immune-histochemistry, and as antigen-binding domains of artificial T cell receptors, etc. Unlike monoclonal antibodies, which are often produced in mammalian cell cultures, scFvs are more often produced in bacteria cell cultures such as E. coli, allowing an easy and faster production of antibodies in massive amounts.
The fragment antigen-binding (Fab fragment) is the antigen-binding region on an antibody, which is composed of a constant and a variable domain. The domains form the paratope — the antigen-binding site — at the amino-terminal portion of the monomer. The two variable domains facilitate binding with specific antigens. For example, Tao Y et al selected for Fabs against Wnt receptors from a phage-displayed synthetic library F [50]. Slezak T et al developed a modular antibody platform based on the phage display of Fab fragments [51].
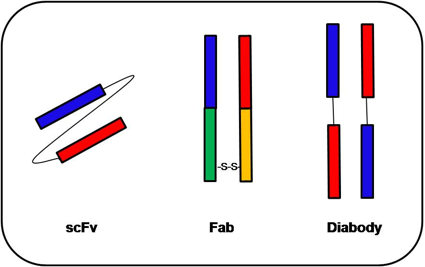
A diabody comprises two scFvs which are connected with linker peptides, too short for the two variable regions to fold together (about five amino acids), forcing dimerization of the scFvs. Diabodies have a much higher affinity to their target, due to much lower dissociation constants.
Another recombinant antibody format, called as scFv-zipper, consists of two scFv antibodies, with same or different antigen specificities, linked with leucine zippers.
Thus, the phage-displayed recombinant antibodies (rAbs), which provide a direct link between the genotype and phenotype of the antibody to be displayed [52], have been displayed as functional binding molecules in different formats of antibody fragments [18, 30]. At present, the most widely used and applicable formats are scFv and Fab fragments, particularly expressed in eukaryotes.
Various types of libraries have been utilized for selection of specific antibodies: (1) antigen- or pathogen-specific library sourced from immunized animals, for example, leukocytes from an immunized llama [42, 53], (2) a single-pot or universal library without any specificity, (3) mutant libraries generated from mutated DNA sequences of a monoclonal hybridoma line or single phage clone [54, 55]. The single-pot universal libraries are extremely assorted and allow the selection of antibody fragments with binding affinities to a wide range of antigens [21] due to the size of their repertoire [56].
Polymerase chain reaction (PCR) has enormously simplified the cloning of variable region genes. The cDNA synthesized from mRNA isolated from B-lymphocyte population is used as a template for PCR- based amplification of VL and VH immunoglobulin genes. The mRNA can also be isolated from hybridoma cell lines, single clones obtained from the primary library. PCR amplification requires oligonucleotide primers complementary to the antibody gene sequences. Degenerate primer sets have been used to amplify almost all the possible sequences for the variable light or variable heavy chain genes for the generation of a library. Many of the primer sets have been reported with various target sequences for primer annealing [57-60].
A set of downstream primers analogous to sequences encoding parts of CH1 and CL chains have been used for amplification of the variable genes from antibodies of different subclasses. Assembly of the two variable domains into a single gene can be made possible by a DNA linker either in the VH-linker-VL format or the VL-linker-VH type [61, 62]. It has been noted that the expression level of scFv in N-termini-VL domain-linker-VH domain C-termini orientation was relatively high in comparison to the other format. Hence, to obtain high yields of the antibodies, insertion of the gene sequence coding for them should be in the desired frame in the phagemid vector. The most significant advantage offered by phagemid vectors is their small size (3–5 kb) and high efficiency for transformation in bacterial cells. In general, the recombinant phagemid is introduced into competent E. coli, followed by infection of the bacterial host cells with a helper phage (M13VCS or K07) to yield a recombinant phage that displays antibody fragments as fusions to one of the phage coat proteins.
Solemani Zadeh et al published a detailed protocol for efficient construction and screening of synthetic domain phage variable libraries (with 50-fold improvement of elution efficiency) [63]. Ferrara F et al combined phage and yeast display to identify monoclonal antibodies with desired binding properties [64].
Phage display based in vitro affinity selection of antibodies resembles the clonal selection of antibodies in vivo [65]. The target antigen is immobilized on surfaces based on the choice of the researcher, and the library is applied onto it, enabling affinity-based selection of highly reactive antibodies. The non-specific phage particles get eliminated during washing steps, and those which remain bound to the immobilized antigen, are eluted by addition of appropriate elution reagents. The antigen-specific phages can then be amplified in a proper strain of E. coli for their downstream applications or the next selection cycle facilitating further enrichment. This affinity selection process is known as bio-panning, and the final round of it can be determined by assessment of no more additional rise in the yield of eluted phages [65].
Bio-panning includes recurring rounds of phage binding to the target, washing to remove non-binding phage and elution steps to get binding phage and re-amplification of the phage pool enriched for specific binding phage. Any other methods which help in separation of specific from non-binding clones can be employed as selection methods. Most commonly in vitro selection method, such as bio-panning on immobilized antigen coated onto paramagnetic beads [66], plastic tubes or Petri dishes [54], columns with an antigen-activated matrix [18], or specifically coated BIAcore sensor chips [56], selection using biotinylated antigen [55] or direct selection on fixed prokaryotic cells respectively mammalian cells [67] have been reported.
A large fraction of non-specific phages get washed, and the phage clones bearing antibodies with specific binding properties to the matrix are eluted by using appropriate elution reagents. The fact that the M13 phage is stable at extremes of temperature and pH has been utilized successfully in experimenting with the choice of elution reagents. Acidic solutions, like HCl, glycine buffers [56, 68], basic solutions, like triethylamine [54], or even reducing agents have been used. In other cases, a competition elution by an excess concentration of antigen [24] or antibodies to the antigen has also been reported. Another gentle strategy has been the use of enzymes for cleavage of a protease site engineered between the antibody and pIII protein of the phage [21].
An in vivo selection involves the introduction of phage particles directly into animals of choice followed by the collection of target tissues. Such a method has helped in the research studies on cellular receptors and identification of peptides or antibodies with specificity for them [69, 70]. For a further selection of monoclonal antibodies, the phage clones obtained after final round are propagated individually and characterized for desired binding specificities [21].
Alfaleh et al, for example, used cell-based biopanning to isolate anti-CD117 antibodies, which were evaluated by flow cytometry and immunohistochemistry [71]. Kim et al panned a Fab phage display library against the spike protein of Middle East respiratory syndrome-coronavirus and generated highly specific Fab fragments and monoclonal antibodies, with the potential as diagnostic agents [72]. D Wrapp et al developed a VHH phage library against the S proteins of MERS-CoV and SARS-CoV-1 viruses using the pMECS vector [73].
The antibodies generated through recombinant methods hold superiority than the hybridoma produced [74], as the former can be improved for its qualities and modified as per the desired applications. Affinity maturation of rAbs has been reported [24, 75]. High-affinity antibodies against a broad spectrum of antigens have been derived from various immunized animals, such as llamas [53, 76], mice [77], chickens [78], rabbits [79], and also from sheep [80] and nonhuman primates [75].
Very high volumetric yields have been reported for cytoplasmic expression of rAbs in E. coli. Production of smaller antibody fragments has been reported up to grams per liter amounts in bacteria [81]. Expression of a functional rAb in E. coli was first reported by Skerra and Plückthun in 1988 [82], where both V chains were translocated into the periplasmic space of the bacterial cells. The oxidizing environment in the compartment allowed correct conformation of the functional Fv fragment by the formation of appropriate disulfide bonds. The approach was also utilized for the expression of first time functional Fab fragments in E. coli [83]. The expression of recombinant antibodies in reducing cytoplasm more often than not results in non-functional aggregates [84].
On the other hand, expression of functional antibody fragments in cytoplasm most of the times requires complete denaturation and refolding [85]. However, successful production of functional scFvs in the cytoplasm of E. coli, not requiring refolding has also been reported [86-88]. The type of host strain and the specificity of the antibodies are known to affect the expression of functional rAbs in E. coli. A good number of rAbs have been produced in the periplasm of E. coli using N-terminal leader sequences targeting the periplasmic Sec pathway [89-91]. Following the expression of the rAbs, the molecules are isolated from the periplasmic fraction [92, 93], or culture supernatants [94, 95]. The expression, periplasmic transport, accurate folding and assembly of two different polypeptide chains is highly necessary for the expression of rAbs in the Fab format. The bi-cistronic vectors with the first cistron encoding the light chain and the second cistron encoding the Fd fragment (consisting of VH and CH1) were found to be optimal for such requirements [92]. Production of a full size glycosylated IgG in E. coli has also been reported [96], only after the fine-tuning of the translation strength of both IgG chains, by the introduction of silent mutations into the translation initiation region of the leader sequence which facilitated optimal periplasmic transport [96].
| Advantages | Disadvantages |
|---|---|
|
|
Hence, high yield expression of rAbs in E. coli can be achieved only after addressing various issues like host toxicity, vector mutation, and plasmid loss. Such decisive constraints can be addressed by the promoter system, plasmid copy number, etc. [97]. One modification is the growth of bacterial cells between the temperature range 20 to 30°C rather than at 37°C to steer clear of the overloading of E. coli’s secretory pathway. Nevertheless, very high yields of antibody fragments in E. coli can be facilitated by high cell density fermentation in bioreactors. Cell-wall-less L-forms of the Gram-negative bacterium Proteus mirabilis have also been experimented for the production of miniAbs and scFvs [98, 99], to obtain high yields of functional scFvs [99].
The expression of recombinant antibody fragments (scFv, Fab) has been reported in numerous microorganisms, especially in selected strains of E. coli. The choice for E. coli host strains such as BL21 and their derivatives amounts to the fact that the antibodies can be produced economically in bulk amounts. The soluble antibody fragments can be expressed in the culture supernatant, bacterial periplasm, and/or inside the bacterial cytoplasm [100-102]. Though the secretion of the rAbs in the periplasm and the media provide a less overall yield, the oxidative environment outside the cell cytoplasm confers proper formation of disulfide bonds required for natural folding of antibody domains.
Antibodies expressed into the periplasmic space are extracted by osmotic shock. RAbs expressed in considerable amounts in the cell cytoplasm frequently results in extensive aggregation and formation of inclusion bodies, which can be purified from the other cellular components. The aggregated antibody mass can be made soluble by adding strong denaturants for instance urea or guanidium hydrochloride including some oxido-reduction system. The native antibody fragments are recovered by refolding upon removal of the denaturants. Guidelines for antibody refolding have been extensively reviewed [103].
Apart from E. coli host cells, the rAbs have been expressed in mammalian systems [104, 105], insect cells [106, 107], yeasts like Saccharomyces cerevisiae [108], Schizosaccharomyces pombe [109], Hansenula polymorpha [110], and also in Pichia pastoris [111] and in bacterial host strains such as Bacillus brevis [112]. Generally, the choice of expression hosts (bacteria, yeast, plants, insect or mammalian cells) must be reliable as per the final application of the antibody molecule.
| Vectors | Antibody format | Library/ antibody host | Antibody source | Target | References |
|---|---|---|---|---|---|
| pCOMB3 & its variants, pCOMB8 | Fab, scFv; zybodies | E. coli XL1-Blue; HEK293F suspension cells; ER2537 cells; SURE cells; | phage lambda library; human bone marrow; hybridoma 6E6A8F3B line; human; immunized BALB/c mice spleen cell RNA; human PBMC RNA; mouse monoclonal anti-Bet v1 antibody; human splenic lymphocytes; | antitetanus toxoid; hepatitis C virus; ErbB2, EGFR, IGF-1R, Ang2 and integrin αvβ3; Burkholderia pseudomallei exotoxin; glycophorin A; Daudi cells human B-lymphoma; H3N2 influenza A virus strains; human B lymphoma cells; human Raji cell strain in B-lymphoma; human TNF-alpha; major birch pollen allergen (Betv1); Puumala hantavirus; alpha-galactosyl epitope | [43] |
| pSEX81 & derivatives | scFv; VHH repertoire | E. coli XL1-Blue | Human; camelid; immunized BALB/c mice spleen cell RNA | dinitrophenol, fluorescein isothiocyanate and 3-nitro-4-hydroxy-5-iodophenylacetic acid; naïve camelid; extracellular domain of N-cadherin | [36, 113] |
| pCANTAB 5 & its variants | scFv; short scFv; single domain VH; diabody; anti-idiotypic scFv; | E. coli TG1; HB2151; supE strain of E. coli TG1 cells | Murine; C3A8 hybridoma cells; monoclonal antibody; hybridoma cell line FMC9; immunized mice spleen cell RNA; human peripheral blood mononuclear cells; scFv DNA; human/ mouse; | epidermal growth factor receptor(EGFR); Saccharomyces and Candida species; TNFα; MCF-7 breast cancer cells; Staphylococcal enterotoxin B; 5-methyl-2'-deoxycytidine; human alpha2b interferon; protective antigen of B. anthracis; human IL-6; telomerase protein; anti-HLA-DR and anti-DOTA scFv; human T14(+) anti-DNA antibodies; MUC-1 mucin molecule | [38-40, 114] |
| pRSET Sfi I/Not I | scFv | E. coli BL21(DE3)pLysS | scFv DNA cloned in pCANTAB 5E vector | nonspecific cross-reacting antigen-95 of human granulocytes and myelopoietic cells | [115] |
| pHEN1 | scFv | E. coli | human umbilical vein endothelial cells | von Willebrand factor A1 | [116] |
- Cavalli Björkman N, Osby E, Lundin J, Kalin M, Osterborg A, Gruber A. Fatal adenovirus infection during alemtuzumab (anti-CD52 monoclonal antibody) treatment of a patient with fludarabine-refractory B-cell chronic lymphocytic leukemia. Med Oncol. 2002;19:277-80 pubmed
- Baert F, Noman M, Vermeire S, Van Assche G, D Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601-8 pubmed
- Plosker G, Figgitt D. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-43 pubmed
- Miller K, Weaver Feldhaus J, Gray S, Siegel R, Feldhaus M. Production, purification, and characterization of human scFv antibodies expressed in Saccharomyces cerevisiae, Pichia pastoris, and Escherichia coli. Protein Expr Purif. 2005;42:255-67 pubmed
- Cabezas S, Rojas G, Pavon A, Alvarez M, Pupo M, Guillen G, et al. Selection of phage-displayed human antibody fragments on Dengue virus particles captured by a monoclonal antibody: application to the four serotypes. J Virol Methods. 2008;147:235-43 pubmed
- Almquist K, McLean M, Niu Y, Byrne G, Olea Popelka F, Murrant C, et al. Expression of an anti-botulinum toxin A neutralizing single-chain Fv recombinant antibody in transgenic tobacco. Vaccine. 2006;24:2079-86 pubmed
- Gram H, Marconi L, Barbas C, Collet T, Lerner R, Kang A. In vitro selection and affinity maturation of antibodies from a naive combinatorial immunoglobulin library. Proc Natl Acad Sci U S A. 1992;89:3576-80 pubmed
- Valjakka J, Hemminki A, Niemi S, Söderlund H, Takkinen K, Rouvinen J. Crystal structure of an in vitro affinity- and specificity-matured anti-testosterone Fab in complex with testosterone. Improved affinity results from small structural changes within the variable domains. J Biol Chem. 2002;277:44021-7 pubmed
- Wu S, Lin Y, Chou J, Lin C. Construction and characterization of a Fab recombinant protein for Japanese encephalitis virus neutralization. Vaccine. 2004;23:163-71 pubmed
- Winter G, Griffiths A, Hawkins R, Hoogenboom H. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433-55 pubmed
- Sidhu S, Fairbrother W, Deshayes K. Exploring protein-protein interactions with phage display. Chembiochem. 2003;4:14-25 pubmed
- Hentrich C, Ylera F, Frisch C, Haaf AT, and Knappik A. Chapter 3 – Monoclonal Antibody Generation by Phage Display: History, State-of-the-Art, and Future. In: Vashist SK and Luong JHT, editors. Handbook of Immunoassay Technologies - Approaches, Performances, and Applications. Elsevier. 2018. p. 47-80.
- Smith G. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315-7 pubmed
- McCafferty J, Griffiths A, Winter G, Chiswell D. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552-4 pubmed
- Burton D. Phage display. Immunotechnology. 1995;1:87-94 pubmed
- Rodi D, Makowski L. Phage-display technology--finding a needle in a vast molecular haystack. Curr Opin Biotechnol. 1999;10:87-93 pubmed
- Willats W. Phage display: practicalities and prospects. Plant Mol Biol. 2002;50:837-54 pubmed
- Ryu D, Nam D. Recent progress in biomolecular engineering. Biotechnol Prog. 2000;16:2-16 pubmed
- Barbas C, Kang A, Lerner R, Benkovic S. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991;88:7978-82 pubmed
- Clackson T, Hoogenboom H, Griffiths A, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624-8 pubmed
- Devlin J, Panganiban L, Devlin P. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404-6 pubmed
- Scott J, Smith G. Searching for peptide ligands with an epitope library. Science. 1990;249:386-90 pubmed
- Sidhu S. Engineering M13 for phage display. Biomol Eng. 2001;18:57-63 pubmed
- Mead DA, Kemper B. In Vectors: A survey of molecular cloning vectors and their uses; Rodriquez, R. L., Denhardt, D. T., Eds.; Butterworths: Boston, 1988.
- Kang A, Barbas C, Janda K, Benkovic S, Lerner R. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci U S A. 1991;88:4363-6 pubmed
- Hoogenboom H, Griffiths A, Johnson K, Chiswell D, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991;19:4133-7 pubmed
- Bass S, Greene R, Wells J. Hormone phage: an enrichment method for variant proteins with altered binding properties. Proteins. 1990;8:309-14 pubmed
- Gao C, Mao S, Lo C, Wirsching P, Lerner R, Janda K. Making artificial antibodies: a format for phage display of combinatorial heterodimeric arrays. Proc Natl Acad Sci U S A. 1999;96:6025-30 pubmed
- Jespers L, Messens J, De Keyser A, Eeckhout D, Van den Brande I, Gansemans Y, et al. Surface expression and ligand-based selection of cDNAs fused to filamentous phage gene VI. Biotechnology (N Y). 1995;13:378-82 pubmed
- Fuh G, Sidhu S. Efficient phage display of polypeptides fused to the carboxy-terminus of the M13 gene-3 minor coat protein. FEBS Lett. 2000;480:231-4 pubmed
- Rondot S, Koch J, Breitling F, Dubel S. A helper phage to improve single-chain antibody presentation in phage display. Nat Biotechnol. 2001;19:75-8 pubmed
- Smith GP. Preface. Surface display and peptide libraries. Gene. 1993;128:1-2.
- McConnell S, Kendall M, Reilly T, Hoess R. Constrained peptide libraries as a tool for finding mimotopes. Gene. 1994;151:115-8 pubmed
- Petrenko V, Smith G, Gong X, Quinn T. A library of organic landscapes on filamentous phage. Protein Eng. 1996;9:797-801 pubmed
- Kishchenko G, Minenkova O, Il ichev A, Gruzdev A, Petrenko V. [Structure of virions of the M13 phage containing chimeric B-protein molecules]. Mol Biol (Mosk). 1991;25:1497-503 pubmed
- Kishchenko G, Batliwala H, Makowski L. Structure of a foreign peptide displayed on the surface of bacteriophage M13. J Mol Biol. 1994;241:208-13 pubmed
- Cesareni G. In Vectors: A survey of molecular cloning vectors and their uses; Rodriquez RL, Denhardt DT, Eds.; Butterworths: Boston. 1988.
- Yau K, Lee H, Hall J. Emerging trends in the synthesis and improvement of hapten-specific recombinant antibodies. Biotechnol Adv. 2003;21:599-637 pubmed
- Marks J, Hoogenboom H, Bonnert T, McCafferty J, Griffiths A, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581-97 pubmed
- Hawkins R, Russell S, Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992;226:889-96 pubmed
- Hoogenboom H, de Bruine A, Hufton S, Hoet R, Arends J, Roovers R. Antibody phage display technology and its applications. Immunotechnology. 1998;4:1-20 pubmed
- Benhar I, Pastan I. Cloning, expression and characterization of the Fv fragments of the anti-carbohydrate mAbs B1 and B5 as single-chain immunotoxins. Protein Eng. 1994;7:1509-15 pubmed
- Ruberti F, Cattaneo A, Bradbury A. The use of the RACE method to clone hybridoma cDNA when V region primers fail. J Immunol Methods. 1994;173:33-9 pubmed
- Larrick J, Danielsson L, Brenner C, Abrahamson M, Fry K, Borrebaeck C. Rapid cloning of rearranged immunoglobulin genes from human hybridoma cells using mixed primers and the polymerase chain reaction. Biochem Biophys Res Commun. 1989;160:1250-6 pubmed
- Sblattero D, Bradbury A. A definitive set of oligonucleotide primers for amplifying human V regions. Immunotechnology. 1998;3:271-8 pubmed
- Tsumoto K, Nakaoki Y, Ueda Y, Ogasahara K, Yutani K, Watanabe K, et al. Effect of the order of antibody variable regions on the expression of the single-chain HyHEL10 Fv fragment in E. coli and the thermodynamic analysis of its antigen-binding properties. Biochem Biophys Res Commun. 1994;201:546-51 pubmed
- Merk H, Stiege W, Tsumoto K, Kumagai I, Erdmann V. Cell-free expression of two single-chain monoclonal antibodies against lysozyme: effect of domain arrangement on the expression. J Biochem. 1999;125:328-33 pubmed
- Petrenko V, Vodyanoy V. Phage display for detection of biological threat agents. J Microbiol Methods. 2003;53:253-62 pubmed
- de Kruif J, Boel E, Logtenberg T. Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol. 1995;248:97-105 pubmed
- Parmley S, Smith G. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988;73:305-18 pubmed
- Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364-6 pubmed
- Johns M, George A, Ritter M. In vivo selection of sFv from phage display libraries. J Immunol Methods. 2000;239:137-51 pubmed
- Verhaar M, Chester K, Keep P, Robson L, Pedley R, Boden J, et al. A single chain Fv derived from a filamentous phage library has distinct tumor targeting advantages over one derived from a hybridoma. Int J Cancer. 1995;61:497-501 pubmed
- Azzazy H, Highsmith W. Phage display technology: clinical applications and recent innovations. Clin Biochem. 2002;35:425-45 pubmed
- Tout N, Yau K, Trevors J, Lee H, Hall J. Synthesis of ligand-specific phage-display ScFv against the herbicide picloram by direct cloning from hyperimmunized mouse. J Agric Food Chem. 2001;49:3628-37 pubmed
- Andris Widhopf J, Rader C, Steinberger P, Fuller R, Barbas C. Methods for the generation of chicken monoclonal antibody fragments by phage display. J Immunol Methods. 2000;242:159-81 pubmed
- Li Y, Cockburn W, Kilpatrick J, Whitelam G. High affinity ScFvs from a single rabbit immunized with multiple haptens. Biochem Biophys Res Commun. 2000;268:398-404 pubmed
- Charlton K, Harris W, Porter A. The isolation of super-sensitive anti-hapten antibodies from combinatorial antibody libraries derived from sheep. Biosens Bioelectron. 2001;16:639-46 pubmed
- Carter P, Kelley R, Rodrigues M, Snedecor B, Covarrubias M, Velligan M, et al. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Biotechnology (N Y). 1992;10:163-7 pubmed
- Skerra A, Pluckthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988;240:1038-41 pubmed
- Better M, Chang C, Robinson R, Horwitz A. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988;240:1041-3 pubmed
- Schmidt F. Recombinant expression systems in the pharmaceutical industry. Appl Microbiol Biotechnol. 2004;65:363-72 pubmed
- Martineau P, Jones P, Winter G. Expression of an antibody fragment at high levels in the bacterial cytoplasm. J Mol Biol. 1998;280:117-27 pubmed
- Proba K, Wörn A, Honegger A, Pluckthun A. Antibody scFv fragments without disulfide bonds made by molecular evolution. J Mol Biol. 1998;275:245-53 pubmed
- Wörn A, Pluckthun A. Mutual stabilization of VL and VH in single-chain antibody fragments, investigated with mutants engineered for stability. Biochemistry. 1998;37:13120-7 pubmed
- Wörn A, Pluckthun A. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS Lett. 1998;427:357-61 pubmed
- Sletta H, Tøndervik A, Hakvåg S, Aune T, Nedal A, Aune R, et al. The presence of N-terminal secretion signal sequences leads to strong stimulation of the total expression levels of three tested medically important proteins during high-cell-density cultivations of Escherichia coli. Appl Environ Microbiol. 2007;73:906-12 pubmed
- Tachibana H, Takekoshi M, Cheng X, Nakata Y, Takeuchi T, Ihara S. Bacterial expression of a human monoclonal antibody-alkaline phosphatase conjugate specific for Entamoeba histolytica. Clin Diagn Lab Immunol. 2004;11:216-8 pubmed
- Rusch S, Kendall D. Interactions that drive Sec-dependent bacterial protein transport. Biochemistry. 2007;46:9665-73 pubmed
- Kirsch M, Zaman M, Meier D, Dubel S, Hust M. Parameters affecting the display of antibodies on phage. J Immunol Methods. 2005;301:173-85 pubmed
- Ward E. Antibody engineering using Escherichia coli as host. Adv Pharmacol. 1993;24:1-20 pubmed
- Lauer B, Ottleben I, Jacobsen H, Reinard T. Production of a single-chain variable fragment antibody against fumonisin B1. J Agric Food Chem. 2005;53:899-904 pubmed
- Mi J, Yan J, Guo Z, Zhao M, Chang W. Isolation and characterization of an anti-recombinant erythropoietin single-chain antibody fragment using a phage display antibody library. Anal Bioanal Chem. 2005;383:218-23 pubmed
- Simmons L, Reilly D, Klimowski L, Raju T, Meng G, Sims P, et al. Expression of full-length immunoglobulins in Escherichia coli: rapid and efficient production of aglycosylated antibodies. J Immunol Methods. 2002;263:133-47 pubmed
- Dubel S, Breitling F, Klewinghaus I, Little M. Regulated secretion and purification of recombinant antibodies in E. coli. Cell Biophys. 1992;21:69-79 pubmed
- Kujau M, Hoischen C, Riesenberg D, Gumpert J. Expression and secretion of functional miniantibodies McPC603scFvDhlx in cell-wall-less L-form strains of Proteus mirabilis and Escherichia coli: a comparison of the synthesis capacities of L-form strains with an E. coli producer strain. Appl Microbiol Biotechnol. 1998;49:51-8 pubmed
- Rippmann J, Klein M, Hoischen C, Brocks B, Rettig W, Gumpert J, et al. Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli. Appl Environ Microbiol. 1998;64:4862-9 pubmed
- Hayden M, Gilliland L, Ledbetter J. Antibody engineering. Curr Opin Immunol. 1997;9:201-12 pubmed
- Dorai H, McCartney J, Hudziak R, Tai M, Laminet A, Houston L, et al. Mammalian cell expression of single-chain Fv (sFv) antibody proteins and their C-terminal fusions with interleukin-2 and other effector domains. Biotechnology (N Y). 1994;12:890-7 pubmed
- Uppala A, Koivunen E. Targeting of phage display vectors to mammalian cells. Comb Chem High Throughput Screen. 2000;3:373-92 pubmed
- Mahiouz D, Aichinger G, Haskard D, George A. Expression of recombinant anti-E-selectin single-chain Fv antibody fragments in stably transfected insect cell lines. J Immunol Methods. 1998;212:149-60 pubmed
- Ailor E, Pathmanathan J, Jongbloed J, Betenbaugh M. A bacterial signal peptidase enhances processing of a recombinant single chain antibody fragment in insect cells. Biochem Biophys Res Commun. 1999;255:444-50 pubmed
- Frenken L, van der Linden R, Hermans P, Bos J, Ruuls R, de Geus B, et al. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J Biotechnol. 2000;78:11-21 pubmed
- Davis G, Bedzyk W, Voss E, Jacobs T. Single chain antibody (SCA) encoding genes: one-step construction and expression in eukaryotic cells. Biotechnology (N Y). 1991;9:165-9 pubmed
- Abdel Salam H, El Khamissy T, Enan G, Hollenberg C. Expression of mouse anticreatine kinase (MAK33) monoclonal antibody in the yeast Hansenula polymorpha. Appl Microbiol Biotechnol. 2001;56:157-64 pubmed
- Marty C, Scheidegger P, Ballmer Hofer K, Klemenz R, Schwendener R. Production of functionalized single-chain Fv antibody fragments binding to the ED-B domain of the B-isoform of fibronectin in Pichia pastoris. Protein Expr Purif. 2001;21:156-64 pubmed
- Shiroza T, Shinozaki Kuwahara N, Hayakawa M, Shibata Y, Hashizume T, Fukushima K, et al. Production of a single-chain variable fraction capable of inhibiting the Streptococcus mutans glucosyltransferase in Bacillus brevis: construction of a chimeric shuttle plasmid secreting its gene product. Biochim Biophys Acta. 2003;1626:57-64 pubmed
- Yi K, Chung J, Kim H, Kim I, Jung H, Kim J, et al. Expression and characterization of anti-NCA-95 scFv (CEA 79 scFv) in a prokaryotic expression vector modified to contain a Sfi I and Not I site. Hybridoma. 1999;18:243-9 pubmed
- Zhu H, Wang Y, Jiang M, Ji S, Bai X, Ruan C. Generation and characterization of a recombinant single chain Fv antibody to von Willebrand factor A1 domain from phage display library. Thromb Res. 2005;116:385-91 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Anti-GPCR Antibodies
- Antibody Applications
- Antibody Companies
- Antibody Conjugation
- Antibody Dilution and Antibody Titer
- Antibody Quality
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- Aptamers and Affimers
- Monoclonal Antibodies: Expression and Purification in a Basic Research Laboratory
- Monoclonal Antibodies - Quality Control and Quantification through Mass Spectrometry
- Mouse Antibody
- Protein Expression
- Rabbit Antibody
- Rat Antibody
- Recombinant Antibodies
- Secondary Antibodies
