Scientists employ a range of methods to investigate neuronal activity and the effect of drugs on neurotransmitter systems. This review introduces the topic of neuronal activity and it discusses commonly used techniques: microdialysis, fast-scan cyclic voltammetry, optogenetics, patch clamp, PET, and functional MRI.
The nervous system processes sensory information and controls behavior. The principal cells that are involved in information processing are neurons, cells that are specialized to receive, process, and transmit information. Information processing is mediated by neurotransmitters, chemical substances that carry the information between neurons and across synapses. Released neurotransmitters diffuse and bind receptors, an event that converts the message into an electrical signal which is then integrated and affects the polarization state of the cell. At rest, in a non-excited state, the neuronal membrane is polarized, bearing a negative charge.
In a classical chemical transmission, a neurotransmitter is released from a presynaptic neuron and binds to receptors on the postsynaptic neuron, often on the dendrites. The chemical signals serve as ligands and activate receptors such as ion channels, and affect the ion flux across the membrane. Depending on which type of ion channels are activated, a hyperpolarization signal may enter the cell when the balance of negative charges exceeds the positive, or depolarization when positive charges exceed the negative.
Since the discovery of the first neurotransmitter, acetylholine, in 1926 by Otto Loewi [7], a large number of neurotransmitters have been discovered. A partial list of neurotransmitters based on their chemical grouping is presented in Table 1 [8]. Defining the experimental criteria for determining which chemical ligand may be considered a neurotransmitter has been challenging, and consequently specific criteria was suggested in order to define a chemical ligand as a neurotransmitter. For example, that presynaptic neurons must contain the appropriate synthetic enzymes to produce the ligand, and postsynaptic cells must contain receptors for the putative neurotransmitters [9]. These criteria facilitated neurotransmitters organization, but challenges remain. The chemical category of gaseous neurotransmitters such as nitric oxide (NO) and carbon monoxide (CO) for example cannot be stored, and are therefore not maintained within synaptic vesicles as most other neurotransmitters.
| Type | Neurotransmitter |
|---|---|
| Monoamines & acetylcholine | Acetylcholine Dopamine Epinephrine Serotonin Histamine |
| Amino acids | Glutamate Aspartate Gamma-aminobutyric acid (GABA) Glycine D-serine |
| Purines | Adenosine Adenosine triphosphate |
| Lipids | Anadamnide (endocannabinoid) 2-arachido noylglycerol (2-AG) endocannabinoid |
| Peptides | Enkephalins Beta-endorphin Dynorphins Substance P Neuropeptide Y (NPY) Peptide YY (PYY) Orexin Vasopressin Oxytocin Somatostatin Neurotensin Galanin Bombesin Bradykinin Vasoactive intestinal polypeptide (VIP) Corticotrophin releasing hormone (CRH) |
| Gases | Nitric oxide (NO) Carbon monoxide (CO) |
Because neurotransmitters play a central role in brain function and neuronal activity, neurotransmitter receptors and other proteins that are involved in neurotransmitters synthesis and inactivation are critical targets for the development of therapeutic drugs. Moreover, drugs that can mimic (agonists) or interfere (antagonists) with the action of neurotransmitters are important in evaluating their potential effects on human diseases and behavior. Specifically, an agonist is defined as a drug or a chemical moiety that mimics the effect of a neurotransmitter and activates the receptor. For example, succinyl choline is an agonist that mimics the activity of the neurotransmitter acetylcholine on nicotinic receptors. An antagonist is defined as an agent that opposes the effect of a neurotransmitter, and prevents the receptor activity, often by blocking the neurotransmitter binding site on its receptors. For example, Curare is an antagonist that blocks acetylcholine from binding to nicotinic receptors.
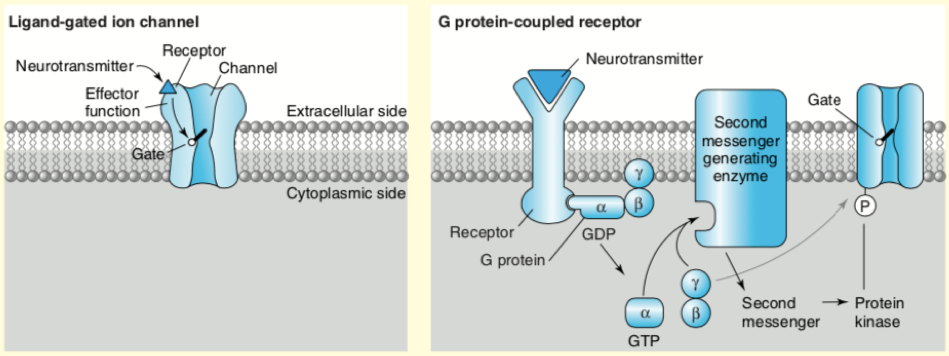
Neuronal receptors are membrane proteins that are activated by neurotransmitters. There are two major types of neurotransmitter receptors: ionotropic and metabotropic. Ionotropic receptors are ion channels that are excited by neurotransmitters such as glutamate and aspartate and inhibited by neurotransmitters such as gamma-aminobutyric acid (GABA) and glycine. Metabotropic receptors are activated by second messengers such as calcium (Ca2+) which relay the message through G protein-coupled receptors and modulate the actions of excitatory and inhibitory ion channels or trigger a signaling cascade that releases Ca2+ from stores inside the cell. Most neurotransmitters receptors are G-protein coupled. Figure 1 below describe the two most common neurotransmitter receptors types; the ligand-gated channels (left), and the G protein-coupled receptors (right). The main difference in these receptors is that they use different mechanisms in converting the neurotransmitter ligand into a cellular signal. The ligand-gated channels act by opening a central pore that permits ions to pass, and the G-coupled receptors act by activating a trimeric G-proteins and their cognate signaling cascades, often by regulating second messenger generating enzymes [8]. G-protein coupled receptor activation may also lead to changes in membrane potential, but the onset is slower and involves several steps in the process when compared with the ligand-gated channel effect. However, by activating protein kinases that phosphorylate ion channels for example, G-protein coupled receptors may alter the responsiveness of neurons to excitatory or inhibitory neurotransmission, and by activating second messenger systems and gene expression, these receptors may initiate functional and structural changes in neurons [10].
The study of neuronal receptor activity is complex, in part because more than one neurotransmitter may activate a receptor. In addition, some neurotransmitters may activate both ligand-gated channels, and G-protein coupled receptors as observed for acetylcholine, glutamate, and GABA [8]. To gain insights into chemical communications that may help establish correlations between the chemical dynamics and behavior, disease progression, and drug effects in intact circuitry, the signaling chemicals and metabolites that alter the neuroreceptors activity must be measured, preferably in the extracellular space of live subjects. This review introduces the different research methods and techniques that are used to investigate neurotransmission.
One question that is being investigated in the field of neuronal receptor activity is by what mechanisms do drugs alter neurotransmission? Such knowledge may help identify the precise step and mechanism that a drug disrupts, and provide crucial insight into its impact on users, and facilitate the development of therapeutic interventions that may inhibit, counter, or reverse a neurological disorder. The methods described below help in answering this important question.
Scientists may perform chemical assays on brain tissues to quantify the presence of a neurotransmitter, receptor, or other structure of interest. The two main assays described in this section are microdialysis and fast-scan cyclic voltammetry. These techniques measure neurotransmitter quantities and fluctuations.
Microdialysis is a popular method for mapping chemical dynamics in brain interstitial tissue fluid of live subjects. This sampling technique allows the assessment of neurotransmitter fluctuations to be related to behavior, drug effects, and disease states. Because neurons communicate by releasing neurotransmitters, measuring signaling chemicals and metabolites in the extracellular space provide important insights into this chemical communication. In addition, microdialysis in awake subjects allows for the correlation between chemical dynamics and behavior, disease progression, and drug effects in intact system [9]. Initial studies were conducted in the laboratory rats to collect samples for the analysis of monoamines and their metabolites. Monoamines are widely distributed throughout the peripheral and central nervous system, and have been implicated in a variety of brain functions, and their dysregulation contributes to a range of neuropsychiatric disorders including Parkinson’s disease, depression, pain, and drug addiction [11]. Many other analytes have been successfully sampled by microdialysis with a broad range of applications including also analyses of large aggregating proteins like amyloid β [12, 13] and tau [14] that are associated with toxic plaque formation in Alzheimer disease.
Neurochemical measurement is a challenge because neurotransmitters rapidly change in concentration, and the extracellular space contains a mixture of components with high chemical complexity [15, 16]. When microdialysis measurements are coupled with additional analytical measurements, the sensitivity and selectivity of low molecular weight neurotransmitters, metabolites, and drugs may also be monitored [17, 18].
Recent technological developments which improve the temporal and spatial resolution of the methods enabled it to be used for monitoring more neurotransmitters simultaneously by liquid chromatography-tandem mass spectrometry (LC-MS/MS). This technique allows multiplexed analysis of neurotransmitters, metabolites, and neuropeptides. This method also improves the separation on reverse phase columns by using reagents that enhance the separation and resolution of neurochemicals in the dialysate samples. For example, benzoyl chloride (BzCl), which reacts with amine and phenol groups, allows for many neurotransmitters to be labeled with increased sensitivity. Consequently, using BzCl with LC-MS/MS has allowed for measurement of as many as 70 neurochemicals in a single assay [19]. In addition, by introducing a drug into the bloodstream via the microdialysis probe, it is possible to carefully monitor the effect of infused drugs or known compounds (retrodialysis). Thus, these advances have allowed for multiplexed neurotransmitter measurements in behavioral, circuit analysis, and drug effect studies.
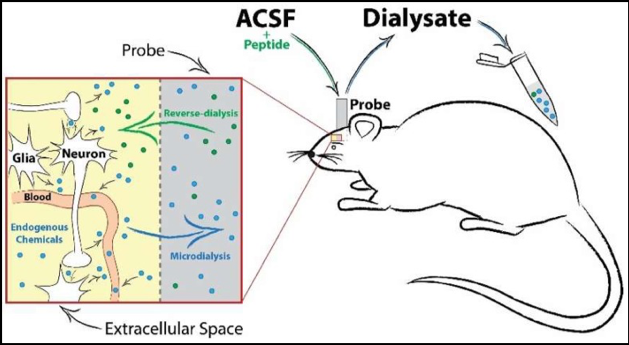
To carry out microdialysis measurement, samples are collected from the brain extracellular space using a sampling probe, such as A-I-4-02 from Eicom [20], - the principal element of microdialysis. Collected fractions are then analyzed for neurotransmitters of interest. A semi-permeable membrane is used as a probe because substances from the extracellular space can cross the membrane according to their concentration gradient. A variety of membranes are available which differ in pore size and the material used. Once the probe is inserted into an external medium (i.e., tissue) and perfusion begins, substances on the outside surface of the dialysis membrane diffuse through the membrane into the probe along their concentration gradient. The addition of antibodies or other affinity agents to the perfusion flow help enhance the concentration gradient and recovery of the neuropeptides and cytokines [9]. Several microdialysis protocols have been published for the assessment of specific substances, and these may be modified according to the investigator’s goals [21]. However, when establishing procedures for the collection of substances not already described in the literature, it is recommended to consider some 11 general key strategies as described by Vladimir C et al [11].
Figure 2 is a schematic of in vivo microdialysis probe setup, neurochemical diffusion, and sample collection. Artificial cerebrospinal fluid (ACSF) is perfused continuously into the probe, creating a concentration gradient at the semi-permeable membrane tip. This allows for the passive diffusion of extracellular transmitters or solubilized compounds or drugs in the ACSF to freely enter or exit the probe. A timeline of collection can then be implemented to investigate changes in neurotransmission before and after an experimental manipulation.
When Arvid Carlsson discovered that dopamine acts as a neurotransmitter in the brain [22], the need for a technique that can help quantify the complexities of redox-active molecules in live brain tissues grew. The first voltammetry experiments that studied the oxidation of catecholamines and other compounds that are associated with neuronal communication in brain tissues were conducted by 1965 [23, 24]. Fast-scan cyclic voltammetry (FSCV) was later developed by Mark Wightman and Julian Millar as a method that follows neurochemical dynamics in real time [25]. FSCV was developed as a means to study chemical communication between cells on a sub-second time scale and it has been applied to many biological studies in a variety of preparations including cells [26], ex vivo tissue slices from the brain and spinal cord, and adrenal glands [27].
The principle device of this method is a small carbon fiber electrode that is inserted into the study sample, and used to quickly raise and lower the voltage in a triangular wave fashion. When the voltage applied is in the correct range (typically ±1 Volt) the molecule of interest is repeatedly oxidized and reduced, an event that results in the movement of electrons in solution which ultimately create a small alternating current (nano amps scale) [28]. It is then possible to subtract the background current created by the probe from the resulting current, and generate a voltage vs. current plot that is unique to each compound. Since the time scale of the voltage oscillations is known, this knowledge can be used to calculate and plot the current in the solution as a function of time. The relative concentration of the compound can then be calculated based on the number of electrons that are transferred in each oxidation and reduction reaction.
Triangular cyclic waveforms have been the most utilized in voltammetry, primarily due to the straightforward nature of scanning through a compound’s redox potential to drive a controlled reaction, and then reverse the chemistry on the return scan. However certain adaptations in FSCV such as in the potential limits, and scan rates to control the surface concentration of a particular chemical species in solution, yield different waveforms that provide increased selective measurements and enable expansion of this technique.
The standard triangular waveform is used by the majority of FSCV researchers. It is the most straightforward to apply and interpret and it provides substantial chemical information. Triangular waveforms are typically utilized for the quantifications of catecholamines, purines, hydrogen peroxide, and shifts in pH [29-31].
N-shaped waveform result from manipulation of the holding potential and the ability to scan across the redox potential for a given analyte. For example, the catecholamine waveform can be transformed with a starting potential of 0.33 V instead of a negative potential [32]. It results in N-shaped waveform and the relative intensity of the peaks reflect that a larger portion of the current is derived from diffusion-controlled electrochemical processes as compared to the typical adsorption-controlled voltammetric detection. Thus, by altering the concentration of adsorbed species and mitigating undesired redox current, increased chemical selectivity or coverage of negatively charged analytes can be achieved. Oxygen and serotonin are analytes that are often targeted for detection with N-shaped waveform [33, 34].
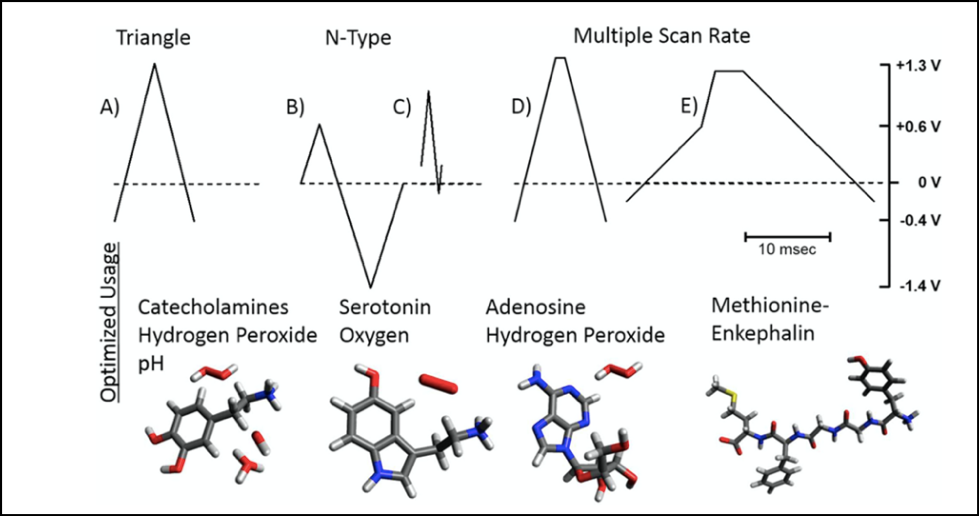
Multiple-scan-rate wave form include a segment during which the potential is held constant while the passage of current is recorded [35]. The desired effect is to remove a layer of adhered material from the electorode surface, maintain reproducible measurements, and gain more chemical selectivity. This approach has been used in the detection of adenosine, among adenosine triphosphate and H2O2, all of which generate a strong oxidation peak near +1.3 V in a triangular waveform on a carbon-fiber microelectorode, thus complicating discrete quantification of these species. When the waveform is altered to contain a segment of zero scan rate, better peak separation is achieved and enhanced features in the voltammograms [36, 37]. Figure 3 describes the different FSCV waveforms and common analytes that are evaluated with each.
Some of the main advantages that are associated with FSCV include the millisecond temporal resolution, a simple two-electrode system, lack of physical sampling requirements, incredibly small size, and low cost. In addition, multiple analytes may be identified by this technique as long as the targeted species exhibit redox activity at distinct potentials. A primary concern in this technique is the chemical selectivity. FSCV cannot always distinguish molecules of a given chemical class. For instance, dopamine, norepinephrine, and epinephrine are all catecholamines that generate similar voltammetric features. Thus, caution must be exercised if these molecules coexist in the same environment, as in adrenal tissue and some regions of the brain. In addition, the analysis of the chemical dynamics data that is recorded in vivo for example uses background subtraction to quantify chemical fluctuations, but a standardized protocol for selecting the most appropriate time point for background subtraction has not been established.
Studies with living animals or people are essential for tying the effects of drugs on neurotransmitters to behaviors or symptoms. These studies are often designed to evaluate the effect of an agonist or antagonist that has a known effect on a particular neurotransmitter, on the subjects’ behavior. Two key techniques in live studies are optogenetics and patch clamp. Optogenetics enables researchers to raise and lower the activity of specific neurons in targeted brain regions in living animals and observe the effects on the animals’ behavior. Patch clamp enables the measurement of the electrical properties of cells in the form of action potentials through the study of ionic currents. In addition, brain scans and electroencephalography (for example, in schizophrenia [38] ) can measure global and regional neuronal activity. Zullo JM et al, for example, implanted mice with wireless telemetry units (PhysioTel ETA-F10) from Data Sciences International to record epileptiform activity [39].
Identification and bioengineering of light-sensitive ion channels (e.g., channelrhodopsins, halorhodopsin, and archaerhodopsins) from the bacteria, have made it possible to use light to artificially modulate neuronal activity in a method named optogenetics. This cutting-edge method is a powerful tool in neuroscience. It allows for remote manipulation of neuronal activity with high spatiotemporal resolution, selective and bidirectional modulation of the activity of defined populations of neurons with great specificity, and it enables significant advances in deciphering how the nervous system works and its influence on various physiological processes in health and disease [40]. The fundamental elements of optogenetics and some of this method’s applications have been described here.
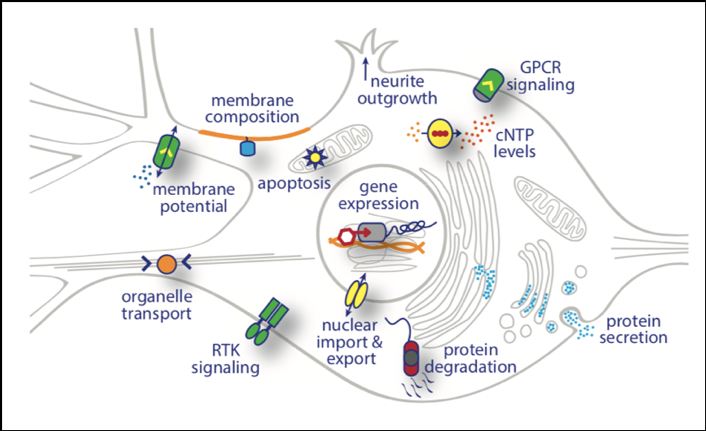
In regard to neuronal activity, the modern optogenetic toolbox includes fluorescent sensors to visualize signaling events in living cells and optogenetic actuators to manipulate multiple cellular activities at specific subcellular targets. These manipulations can match the speed of electrical neuronal activity and essentially offer full control over neuronal activity using closed-loop optogenetics [41]. Figure 4 illustrates the different subcellular targets of optogenetic tools in a neuronal cell body. As indicated, several cellular parameters may be interrogated with photosensitive molecules including membrane potential, membrane composition, intracellular signaling cascades, gene expression, protein and organelle localization, protein secretion, protein degradation, neurite outgrowth, and cell survival [3].
For example, when sensors such as synaptophysin are fused to the cytosolic regions of vesicular proteins, they are not affected by exo and endocytosis and serve as powerful tools for targeting optical sensors to the presynaptic cytosol. Dreosti et al fused GCaMP2 to the cytoplasmic C terminus of synaptophysin, and created the first genetically encoded presynaptic Ca2+ indicator [42]. This strategy was also employed to develop a quantitative genetically encoded presynaptic ATP sensor (Syn-ATP) that enables ratiometric luminescence-to-fluorescence imaging and reporting of presynaptic ATP level changes that are generated by neuronal firing [43]. With multiple targeting systems, it is now possible to perform multiparametric imaging on synaptic contacts using genetically encoded, spectrally distinct sensors such as the pre- or postsynaptically targeted (green fluorescent calmodulin and Ca2+ binding peptide (GCaMPs), which in combination with red-shifted vesicular pH sensors, indicate exocytosis [44-46]. GCaMPs have become a popular indicator for neuronal activity [39, 47, 48]. Siciliano CA et al, for example, injected a virus encoding Cre-dependent GCaMP6m (AAV5-CAGFLEX-GCaMP6m) into medial prefrontal cortex and retrogradely-travelling canine adenoviral vector CAV2-Cre into dorsal periaqueductal gray to achieve projection-specific neuronal activity monitoring in mice [49]. Lee D et al used GCaMP to identify specific neuronal cell types and employed photoactivatable mCherry (PAmCherry) to permanently label them in a labeling technique termed physiological optical tagging sequencing (PhOTseq) [50].
Optogenetic actuators have also been targeted to presynaptic terminals by fusion to synaptic vesicle (SV) proteins. Rost et al generated the fusion construct, "pHoenix", a light-driven acidification of SVs, in combination with pHluorin-based readout of vesicular pH [51]. This construct, was enriched at presynaptic terminals and localized as a bona fide SV protein, and its activation results in accumulation of protons in synaptic vesicles, providing an additional driving force for vesicular transmitter loading and acutely increased transmitter release. Inactivation of vesicular H+-ATPases, this construct could functionally substitute for endogenous H+ pumps, and enable precise light-controlled vesicular acidification and transmitter uptake.
Light-controlled inactivation of transmitter release is another type of optogenetic interference with presynaptic function that presents an excellent tool for studying the role of individual synapses or synaptic circuits in neuronal computation and behavior. Two prominent strategies have been utilized to achieve synaptic inhibition. In the first, inhibition of synapses based on chromophore-assisted light inactivation (CALI), enabled light-induced ablation of synaptic transmission via local generation of reactive oxygen species (ROS) [52]. These ROS in turn inactivated the surrounding proteins via oxidation of tryptophan, tyrosine, histidine, cysteine, and methionine residues [53]. This method allowed for synaptic inactivation in dissociated neuronal cultures, slice cultures, and in C. elegans neurons in vivo and was partially reversible within 24 hr. The other approach to synaptic inhibition involved replacing endogenous synaptotagmin in C. elegans with synaptotagmin fused to a photosensitive portion of the protein that is important in its regulation [54]. Worms expressing this construct and exposed to blue light, exhibited impaired mobility within 15 min. The downside of these methods is their slow kinetics, highlighting the need to develop alternative methods for synapse inactivation by optogenetic
Remarkably, Sinnen et al developed a new optogenetic approach to modify the molecular composition of the post synaptic membrane, a method for photo-tagging postsynaptic sites with heightened spatiotemporal control. By acutely manipulating the post-synaptic density (PSD) on a fast timescale, and controlling the optogenetic GluA1 positioning, they demonstrated that controlled incooperation of additional GluA1 into the PSD enhanced the excitatory input by unmasking previously silent postsynaptic sites rather than increased synaptic strength [55].
Patch clamp is the gold-standard method to measure the electrical properties of cells in the form of action potentials by studying ionic currents. Action potentials from diverse neurons across the brain form the basis of the neuronal code. When the membrane potential (Vm) is depolarized beyond the threshold, an action potential results, and the biophysical conductance underlying the action potential may be converted to a quantitative signal. Action potential firing in sensory brain areas often correlates with specific features of sensory input, whereas spiking in motor areas often precedes and correlates with movement. Action potential firing are accompanied by calcium influx through voltage-gated calcium channels, and imaging of calcium-sensitive fluorophores can yield important measures of spiking activity in neuronal networks with single-cell resolution [56, 57]. Methods for this technique have been developed to simultaneously measure the action potential firing of many neurons using silicon probes with a high density and large number of recording sites [58, 59]. A detailed protocol for this method has been published in another Labome article which can be found here.
Brain imaging techniques allow for direct assessment of neurotransmission in people and in living animals. Some of the imaging techniques that are used to monitor metabolic activity in selected regions of the brain are positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI). Utilizing the knowledge that each neurotransmitter has a unique distribution among the brain regions, information on locations with modified concentrations provide important clues as to which neurotransmitter is affected under the conditions of the study. For example, researchers using the PET technique may be able to correlate a drug’s transit through the brain with changes in a target neurotransmitter, or, they may stimulate a drug-related behavior and correlate the neurotransmitter fluctuations with the changes in its intensity.
Positron-emission tomography (PET), is a non-invasive, nuclear medicine functional imaging technique, that is used to image neuroreceptor targets and receptor binding in vivo in the brain with high specificity. The system uses a positron-emitting radiograph to detects pairs of gamma rays. The most commonly used radioligand in PET is fluorine-18, which is introduced into the body on a radioactive tracer, a biologically active molecule.
PET neuroimaging assumes that areas of high radioactivity are associated with increased brain activity through measurements of blood flow to different parts of the brain. Zullo JM et al, for example, measured the neuronal activity in mouse brains with a small animal PET/CT scanner (eXplore Vista) from GE Healthcare and the 18fluorodeoxyglucose (FDG) tracer [39]. Several radiotracers (radioligands) have been developed for PET for specific neuroreceptor subtypes. Table 2 provides a summary of PET radiotracers that have demonstrated sensitivity to neurotransmitter release [4, 60]. These are now available for any targets in the brain including various receptor types.
| Target receptors | Labeled compounds | Agonist radiotracers | Antagonist radiotracers | |
|---|---|---|---|---|
| Dopamine Receptors | Excitatory D1-like receptors (D1/D5), inhibitory D2-like receptors (D2/D3/D4) | 11C, 18F, and derivatives [11C]raclopride, [11C]FLB457, [18F]fallypride | For D2/D3: [11C]PHNO | For D1: [11C]SCH23390, [11C]NNC-112 |
| Seratonin System | Seratonin transporters | Transporter occupancy is determined by [11C]DASB, endogenous serotonin release by 5-HT1B receptor tracers;[11C]P943, [11C]AZ10419369 | 5-HT2A receptor tracer [11C]Cimbi-36 | 5-HT4 receptor tracer [11C]SB207145 |
| Noradrenaline receptors | a2 adrenoreceptors | Noradrenaline surrogate marker release in different brain regions with [11C]yohimbine | For α2C adrenoceptors [11C]ORM-13070 | |
| Acetylcholine receptors | Metabotropic muscarinic receptor (mACh), ionotropic nicotinic receptors (nACh) | 11C-labelled (+)3-MPB, (+)3-EPB, (+)3-PPB, and NMPYB | For M2 mACh receptor, [18F]FP-TZTP. For α4β2 and α7 subtype nACh receptors, 2-[18F]FA and 6-[18F]FA | |
| Glutamate and GABA transmission | Tracers bind to allosteric site on a receptor and act as allosteric modulators of binding affinity | For N-methyl D-aspartate (NMDA) receptor, mGlu5 receptor, and GABA levels | For NMDA, [11C]GMOM, [11C]-CNS5161, [18F]GE-179 For mGlu5, [11C]ABP688, GABA levels with ionotropic GABAA and benzodiazepine binding sites such as [11C]flumazenil, and [11C]Ro15-4513 | [11C]Ro15-4513, a partial inverse agonist at the benzodiazepine binding site. |
| Opioid system | G-protein coupled opioid receptors and sigma receptors (D1, D2) | [11C]carfentanil, (S)-(−)- and (R)-(+)-[18F]fluspidine | [11C]diprenorphine | |
| Histamine-Neuropsychiatric disorders | H3 Receptors, that inhibit the release of histamine and other neurotransmitters | [11C]TASP0410457, [11C]MK-8278, [11C]GSK189254 |
To elicit neurotransmitter release for the PET measurements, pharmacological interventions with acute drug stimulation is used in order to change the receptors availability and occupancy and affect the synapse and downstream signaling. The fastest and most effective method to administer a drug is by intravenous injection of a liquid form of the drug (i.v.). Alternatively, oral administration may be used, however, in the latter scenario the time line may be several hours before the PET imaging scan may be acquired. Finally, inhalation may also be used to evaluate neurotransmitter releasing drugs, as in the case of nicotine through cigarette smoking [61-63]. By using exogenous drugs that are in direct competition with the radiotracer (direct agonist or antagonist), specific binding may be evaluated. Such blocking studies are carried within the drug therapeutic window, a range of doses between minimum effective concentrations (MEC) to the minimum toxic concentration (MTC) that produces therapeutic response without causing any significant adverse effect in patients [64]. One important advantage of PET imaging is that it has very high biochemical specificity, such that quantification of in vivo biochemistry is possible. Its sensitivity is in the picomolar range (10−11 to 10−12 mol/L) [64].
While PET provides insight into neurotransmitter or drug binding at specific receptor systems, and it has become the gold standard for imaging glucose metabolism in clinical application in oncology, it lacks anatomical information and spatial and temporal resolution. To investigate the link between neuroreceptor-specific activation and the downstream effects on hemodynamics - a measure of brain function, PET measurements are often complemented with functional magnetic resonance imaging (fMRI). The development of fMRI techniques has advanced behavioral and translational neuroscience by extending traditional anatomical brain imaging to include maps of human brain function. Together, PET and fMRI signal modulation allow for simultaneous measurement and mapping of specific neurochemistry, functional physiology, and the underlying anatomy may be correlated to advance our understanding of in vivo receptor-specific signal modulation at the whole-brain level.
fMRI measures brain activity by detecting changes that are associated with blood flow. This technique relies on the fact that cerebral blood flow and neuronal activation are coupled; i.e., when an area of the brain is active, the blood flow to that region also increases. Specifically, this method primarily uses the blood-oxygen-level-dependent (BOLD) contrast, which maps neural activity in the brain or spinal by imaging the change in blood flow (homodynamic response) that is related to energy use by brain cells. This measure may also be compared to baseline BOLD responses in resting state (task less) to establish the specific BOLD variance. For example, Duncan A et al investigated the chemogenetic stimulation of the medial habenula-interpeduncular nucleus circuit in rats by functional magnetic resonance imaging with a Bruker Biospec 70/30 7 Tesla scanner [65].
Several studies have illustrated the importance of simultaneous acquisition of both PET and fMRI to directly correlated neuroreceptor occupancy with pharmacologically induced changes in functional signaling. In a series PET/fMRI studies using [11C]raclopride, the effects of D2/D3 specific agonist and antagonist challenges were tested to characterize receptor function in non-human primates. These studies revealed a correlation in anatomical space and with dose and demonstrated that it is possible to determine functionality of receptors in a dose-response pattern [66, 67]. Specifically, an inhibitory agonist elicits a decrease in cerebral blood volume (CBV), and an antagonist elicits an increase in CBV that is dose-dependent to receptor occupancy. Together, these animal data helped establish the link between neuroreceptor-specific activation and the downstream effects on hemodynamics.

Figure 5 indicates (left panel) that PET analyses can provide insight into neurotransmitter or drug binding at specific receptor systems as shown in the dose-dependent D2/D3 receptor binding of an agonist with PET tracer [4]. fMRI (right panel) can measure the functional response that is related to the same receptor and stimulus. Together, PET/fMRI measurements can be compared to understand any correlations or discrepancies in the functional signals.
PET and fMRI allow for a more in-depth understanding of receptor dynamics through the combination of dynamic receptor occupancy studies using PET, along with the rapid physiological measures of neurovascular coupling with the MRI signals which are even capable of quantitatively mapping receptor internalization rates in vivo [67]. Together fMRI/PET can provide a unique framework for developing quantitative assays of dynamic dopamine release and dopamine receptor and transporter trafficking in near real time. To accurately quantify the dynamic of PET signals, appropriate dynamic kinetic analysis models must be developed. Consequently, models that include a dynamic association rate as part of the kinetic model have already been developed [68, 69]. These were successfully applied for the detection of dopamine release or exogenous D2 receptor binding using the radiotracer [11C]raclopride for evaluating cigarette smoking [61] or drug injections [67].
In clinical research studies, neuroreceptor PET/MRI approach is envisioned to play an important role in deciphering the mechanisms underlying brain dysfunction, and in diagnosing and personalizing therapy. To avoid drugs side effects, optimal drug dosing strategies could be assessed with PET/fMRI. Finally, a more holistic understanding of brain function and dysfunction in health and disease may be accomplished by investigating for example the functional effects of neurotransmitter overload or deficiency in psychiatric disease.
Genetic tools provide a powerful approach to establishing causal relationships between the activity of specific neuronal circuits and behavior, and insights into drugs effect on neurotransmission. The three main strategies in genetic studies are: to genetically modify light-activated optogenic tools such as the microbial rhodopsins halorhodopsin and archaerhodopsin and other indicators, to utilize Drosophila mutants, or develop chemogentic tools [70].
Optogenic tools were already described above. The development of genetic modifications in the microbial rhodopsins halorhodopsin and archaerhodopsin [71-73] provided researchers with a wider variety of ion-pumping microbial rhodopsins [74], and anion-conducting channelrhodopsins [75-77].
Research in the fruit fly Drosophila melanogaster has led to insights in neural development and has broad implications for neuroscience. Drosophila is currently the eukaryotic model organism that permits the most sophisticated in vivo manipulations of neurons and neuronally expressed genes. This is because the fly nervous system contains a manageable number of neurons with a great diversity of neuronal types that are capable of producing complex behaviors and yielding insights that are relevant across biological systems. The brain of an adult fruit fly is capable of producing a wide range of coordinated behavioral sequences in response to current sensory stimuli and previous experiences. Consequently, Drosophila research has contributed to our understanding of nervous system development [81, 82], growth cone guidance and target recognition [83], exocytosis and endocytosis at synapses [84], and synapse remodeling [85]. Essentially two strategies are utilized in these genetic studies: a neuron-centric and a gene-centric approach.
The neuron-centric approach is based on techniques that labels subsets of neurons, permits removal of specific neurons, impairs neuronal function or increases neuronal activity, followed by assaying an output, such as a specific behavior [86-89]. Just as the role of a gene in a particular process can be assayed by examining the measurable consequences – phenotypes that are associated with its removal, the role of a given neuron in a behavior is assayed by silencing or killing it. Lesion studies in the fly are used to correlate behavioral function to areas of the vertebrate brain. Systematic elimination or silencing of groups of neurons produce a map of brain regions and neurons that are critical for different behaviors that will pave the way for understanding how specific neurons encode and transform information. An alternative approach to silencing neurons is disruption of membrane depolarization. Neurons open voltage-gated sodium channels (encoded by para) in response to membrane depolarization to propagate action potentials. This leads to reduction in the number of sodium channels directly [90] or blocked para conductance with tethered toxins [91]. Most commonly this can be achieved by increasing the potassium conductance, which lowers the resting membrane potential or shunts the current to prevent depolarization [84, 86, 92, 93].
The gene-centric approach is based on forward or reverse genetic methods. Forward genetic screens allow the unbiased identification of novel gene players. This approach places a focus on phenotypic driven identification of mutations in genes involved in a certain biological process such as axon guidance, synaptic transmission, or behavior [94]. Forward genetic screens based on transposon mutagenesis for example are used to identify new genetic loci that affect neuronal features based on P elements [94] and piggyBac [95]. The most productive strategy in identifying new genetic loci is to screen existing collections of transposon insertions [96-98].
Reverse genetic approaches are designed to affect a gene of interest. This approach is driven by an interest in a particular gene, and in the technology that allow selective disruption of this gene [96, 99, 100] by one of five main strategies: transposon excision, altering transposons inserted in the gene, RNA interference (RNAi), and gene targeting through either homologous recombination or zinc finger nucleases. Forward and reverse genetics allow the assessment of phenotypes that are associated with these mutations to provide a better understanding of the role of genes and their corresponding proteins in the nervous system in vivo. Subsequently, gene products can be labeled with protein tags that permit protein visualization. The ability to make specific mutations in neural genes and label particular proteins to determine their cellular and subcellular locations within neurons makes Drosophila an impressive model for the study of the cell biology of neurons.
Chemogenetics has been defined as a method by which macromolecules are engineered to interact with previously unrecognized small molecule chemical actuators [101]. These include nucleic acid hybrids [102], kinases [103], a variety of metabolic enzymes [104, 105] and G-protein coupled receptors (GPCRs) such as Designer Receptors Exclusively Activated by Designer Drugs DREADDs [106-108]. Chemogenetic tools are reviewed in the following references: [75, 101, 109-111].
Among these chemocogenetic tools, GPCRs stand out as targets for many of the pharmaceutical companies to cure and alleviate symptoms of diseases that involve all tissues of the body [112]. More specifically, DREADDs, are used to explore treatment options for various neurodegenerative and psychological conditions that involve processes that occur within and outside the nervous system involving neurotransmitters such as GABA and Glutamate [113].
As indicated in figure 1 above, GPCRs are coupled to a heterotrimeric G protein that is composed of three unique subunits (α, β, and γ) that are membrane bound. The G-α subunit contains a GTPase domain, which is capable of hydrolyzing GTP to GDP. When bound to GDP, the complex is functionally inactive. Upon ligand binding to the GPCR, structural conformational changes produce the release of GDP from the heterotrimeric complex, allowing GTP to bind to the G-α subunit. In this GTP-bound form, the G-α subunit dissociates from the G-β and G-γ subunits and proceeds to interact with its downstream cognate targets to affect a particular signal response, depending upon the GPCR and the specific G-α subunit isoform [114, 115]. A clear understanding of the implications of this extended and modified ternary complex model is important for understanding how GPCR-based chemogenetic technologies can be harnessed in neuroscience.
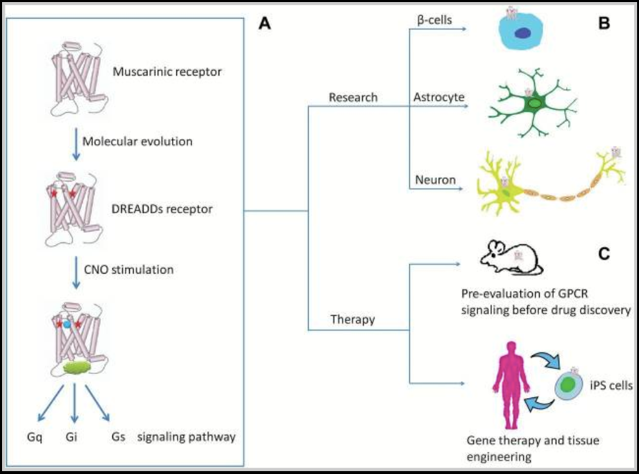
To control GPCR signaling for neuropsychopharmacological therapeutics for example, “selective” agonists or antagonists are typically developed [116, 117]. The essential goal with these tools is to study the regulation of a specific GPCR-signaling pathway in a selective cell population by known pharmacological approaches. However, because most neuronal cells express multiple GPCRs and most GPCRs are expressed in multiple tissues [118], this goal is essentially impossible. Additionally, the presence of endogenous receptor ligands and the lack of true specificity of pharmacological probes for GPCRs further complicate the neuropsychopharmacological approach. To overcome these inherent problems associated with GPCR therapeutic approaches, the GPCR receptors are designed to only respond to a particular drug/ligand, and the drug/ligand to only target that receptor. The technique allows researchers to control the neural activity and study the specific GPCR signaling pathway (Gq, Gi, and Gs) that is associated with a specific GPCR. As indicated in the GPCR signaling in vivo example presented in Figure 6, by introducing two mutations in the transmembrane domains III and V of the muscarinic receptors (noted as red stars), DREADDs receptors that can precisely control the Gq-, Gi-, or Gs-signaling pathways are generated (A). The DREADDs technology can then be applied in a variety of cells including β-cells, astrocytes, and a variety of neurons to control GPCR signaling in vivo (B). The DREADDs technology can then be used in drug discovery, gene therapy, and tissue engineering applications (C) [5].
The engineered GPCR receptors are delivered to the area of interest via viral transduction to adjust the levels neurotransmitters in specific neuron while minimizing the side effects of the treatment or expressed through inducible promotors [47].
The method is very similar to optogenetics (described above), however, it uses chemically engineered molecules and ligands instead of light and light-sensitive channels to understand the relationship between brain activity and behavior. The main difference between these methods is that chemogenetic tools use chemically engineered receptors and exogenous molecules specific for those receptors, to affect the activity of those cells. Because this approach does not require light usage, it provides the benefit of a higher spatial resolution, and is overall favored over optogenetics [119]. Over the past two decades, a large number of chemogenetic platforms have been invented that have been useful for biologists in general and most especially for neuroscientists.
DREADDS specifically have been used in many animal models (e.g., mice and other non-primate animals) to target and influence the activity of various cells [120]. These studies provide human disease models where scientists could determine whether viral expression of DREADD proteins, both in-vivo enhancers and inhibitors of neuronal function, could be used to bidirectionally affect the behaviors and the activity of the involved neurons. Recent studies showed that DREADDs were successfully used to treat/study the motor deficits of rodents modeling Parkinson's disease [121, 122], and in linking the usage of DREADDs and influencing drug seeking and drug sensitization behavior [113]. Zhang X et al injected AAV2/9-hsyn-DIO-GCaMP6m, AAV9-EF1α-DIO-hM3D(Gq)-mCherry and AAV9-EF1α-DIO-hM4D(Gi)-mCherry into the central nucleus of the amygdala and the paraventricular nucleus in mice to study the neuronal control of humoral immune responses in spleen [123]. Bai L et al injected AAV9-DIO-hM3D-mCherry into the nodose ganglion of specific Cre mice and treated the mice with clozapine-N-oxide (CNO) to investigate the role of vagal sensory neuron type in feeding modulation [124]. Duncan A et al used one of DREADDS, M3Dq, and CNO from Enzo, to dissect the role of the medial habenula-interpeduncular nucleus circuit in connecting nicotine addiction to diabetes in rats [65]. Szőnyi A et al employed AAV2/8-hSyn-DIO-hM3D(Gq)-mCherry from Addgene [125]. H Qian et al applied an engineered inhibitory muscarinic receptor variant M4Di in response to CNO to study the newly converted neurons [126].
While chemogenetic tools have widespread utility in animal models [127] and therapeutic applications [128, 129], human therapies would be facilitated by chemogenetic receptors that are potently activated by existing clinically approved drugs. The chemogenetic GPCR tools often rely on GPCR over-expression [101] to achieve high potency responses, and effects on cell electrical activity are variable due to indirect coupling to ion channels.
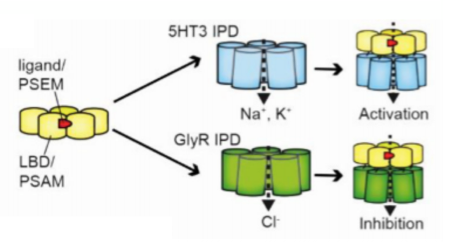
Pharmacologically Selective Actuator Modules (PSAMs) are a modular chemogenetic platform based on modified alpha7 nicotinic acetylcholine receptor (α7 nAChR) ligand binding domains (LBDs) that are engineered to selectively interact with brain-penetrating synthetic agonists called Pharmacologically Selective Effector Molecules (PSEMs).
PSAMs can be combined with various ion pore domains (IPDs) from different ion channels to produce chimeric ligand gated ion channels (LGICs) with common pharmacology but distinct functional properties (Fig. 7). For example, PSAM-5HT3 chimeric channels are cation-selective and PSAM-GlyR channels are chloride-selective, leading to neuron activation or silencing, respectively, in response to PSEM agonists [130].
Engineered chimeric channels have been used to investigate the involvement of specific neuron populations in multiple functions [127]. The primary limitations of PSEMs are short clearance times (30–60 min) and low micromolar potency [130], which are not ideal for in vivo applications. In addition, the sensitivity of PSAMs to clinically approved drugs are unknown.
Christopher Magnus et al combined structure-guided ion channel engineering with synthetic chemistry [6] and developed an ultrapotent chemogenetic system for clinical application. Specifically, the authors synthesized subnanomolar-potency agonists, called uPSEMs, with high selectivity for the chemogenetic receptors and they characterized these in the brains of mice and a rhesus monkey by in vivo electrophysiology, calcium imaging, positron emission tomography, behavioral efficacy testing, and receptor counterscreening [6].
The newly developed ion channel-based platform for cell activation and silencing is controlled by low doses of the smoking cessation drug varenicline. This engineered ion channel technology facilitates chemogenetic therapies because it uses a well-tolerated and approved FDA drug that can potentially be used at or below doses for which it is currently approved. Accordingly, the authors demonstrated chemogenetic perturbations in two nodes of the basal ganglia that are associated with invasive Parkinson’s Disease deep brain stimulation therapies.
These chemogenetic technologies offer opportunities in basic research and the capability for extending findings to potential therapeutic applications. In a recent Cell publication, Rong Gong et al investigated neuroal processes of ingestive behaviors by probing glutamatergic neurons in the peri-locus coeruleus (periLC VGLUT2 neurons) node from energy sensitive (hunger) and hydration-sensitive (thirst) cell populations [131]. The authors also used a PSEM system to image stable hindbrain calcium in free-moving mice, which show that periLC VGLUT2 neurons are tuned to ingestive behaviors and respond similarly to food or water consumption.
This platform of receptors and selective ultrapotent agonists enables potential research and clinical applications of chemogenetics.
To understand how the brain relates to behavior, it is essential to record neural activity. To achieve this goal, a large variety of methods and techniques have been developed to monitor and record the series of events following neuronal firing, including action potentials, intracellular calcium rise [132], neurotransmitter release and immediate early gene expression. This review covered some of the key experimental tools and techniques that scientists use to study drug effects on neurotransmission and their consequences in detecting and integrating neuronal activity in several platforms.
- Karczmar A. The Otto Loewi Lecture. Loewi's discovery and the XXI century. Prog Brain Res. 1996;109:1-27, xvii pubmed
- Hyman S. Neurotransmitters. Curr Biol. 2005;15:R154-8 pubmed
- Trachtenberg J, Chen B, Knott G, Feng G, Sanes J, Welker E, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788-94 pubmed
- Cirrito J, Yamada K, Finn M, Sloviter R, Bales K, May P, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913-22 pubmed
- Pecina S, Smith K, Berridge K. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500-11 pubmed
- Smith K, Berridge K. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594-605 pubmed
- Darvesh A, Carroll R, Geldenhuys W, Gudelsky G, Klein J, Meshul C, et al. In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opin Drug Discov. 2011;6:109-127 pubmed
- Watson C, Venton B, Kennedy R. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391-9 pubmed
- Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490-3 pubmed
- Clark L, Lyons C. Studies of a glassy carbon electrode for brain polarography with observations on the effect of carbonic anhydrase inhibition. Ala J Med Sci. 1965;2:353-9 pubmed
- Hawley M, Tatawawadi S, Piekarski S, Adams R. Electrochemical studies of the oxidation pathways of catecholamines. J Am Chem Soc. 1967;89:447-50 pubmed
- Millar J, Stamford J, Kruk Z, Wightman R. Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. Eur J Pharmacol. 1985;109:341-8 pubmed
- Leszczyszyn D, Jankowski J, Viveros O, Diliberto E, Near J, Wightman R. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem. 1990;265:14736-7 pubmed
- Swamy B, Venton B. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst. 2007;132:876-84 pubmed
- Yuste R, Katz L. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333-44 pubmed
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319-24 pubmed
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446-51 pubmed
- Alpert N, Badgaiyan R, Livni E, Fischman A. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. Neuroimage. 2003;19:1049-60 pubmed
- Han X, Boyden E. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299 pubmed
- Zhang F, Wang L, Brauner M, Liewald J, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633-9 pubmed
- Dickson B. Molecular mechanisms of axon guidance. Science. 2002;298:1959-64 pubmed
- Collins C, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35-42 pubmed
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401-15 pubmed
- Lai S, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703-9 pubmed
- St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3:176-88 pubmed
- Bellen H, Levis R, Liao G, He Y, Carlson J, Tsang G, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761-81 pubmed
- Matthews K, Kaufman T, Gelbart W. Research resources for Drosophila: the expanding universe. Nat Rev Genet. 2005;6:179-93 pubmed
- Adams M, Sekelsky J. From sequence to phenotype: reverse genetics in Drosophila melanogaster. Nat Rev Genet. 2002;3:189-98 pubmed
- Venken K, Bellen H. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167-78 pubmed
- Strobel S, Ortoleva Donnelly L, Ryder S, Cate J, Moncoeur E. Complementary sets of noncanonical base pairs mediate RNA helix packing in the group I intron active site. Nat Struct Biol. 1998;5:60-6 pubmed
- Bishop A, Shah K, Liu Y, Witucki L, Kung C, Shokat K. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8:257-66 pubmed
- Collot J, Gradinaru J, Humbert N, Skander M, Zocchi A, Ward T. Artificial metalloenzymes for enantioselective catalysis based on biotin-avidin. J Am Chem Soc. 2003;125:9030-1 pubmed
- Haring D, Distefano M. Enzymes by design: chemogenetic assembly of transamination active sites containing lysine residues for covalent catalysis. Bioconjug Chem. 2001;12:385-90 pubmed
- Strader C, Gaffney T, Sugg E, Candelore M, Keys R, Patchett A, et al. Allele-specific activation of genetically engineered receptors. J Biol Chem. 1991;266:5-8 pubmed
- Coward P, Wada H, Falk M, Chan S, Meng F, Akil H, et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci U S A. 1998;95:352-7 pubmed
- De Pergola G, De Mitrio V, Perricci A, Cignarelli M, Garruti G, Lomuscio S, et al. Influence of free testosterone on antigen levels of plasminogen activator inhibitor-1 in premenopausal women with central obesity. Metabolism. 1992;41:131-4 pubmed
- Sjulson L, Cassataro D, Dasgupta S, Miesenbock G. Cell-Specific Targeting of Genetically Encoded Tools for Neuroscience. Annu Rev Genet. 2016;50:571-594 pubmed
- Samama P, Cotecchia S, Costa T, Lefkowitz R. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625-36 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Adeno-Associated Viral-Mediated Gene Transfer
- Behavioral Phenotyping in Rats and Mice
- Glial Cell Markers
- Laboratory Mice and Rats
- NMR in Biomedical Research
- Neuronal Cell Markers
- Neuronal Receptor Agonists and Antagonists
- Optical Clearing of Biological Tissue
- Optogenetics
- Patch Clamp Protocol
- Receptor-Ligand Binding Assays
- Retrograde Tracing