An overview of the nanodiscs technology in membrane biology.
Nanodiscs are membrane mimics that can be used to hold membrane proteins in a soluble form for a variety of different downstream applications, including cancer diagnosis and treatment [1]. As their name suggests, nanodiscs are disc-shaped, nanoscale, phospholipid bilayers surrounded by two molecules of an amphipathic alpha-helical protein that surrounds the bilayer disc like a belt and limit its size (Figure 1) [2].

Many membrane proteins have been incorporated into nanodiscs, including P-glycoprotein [3, 4], a number of G-protein-coupled receptors (apelin receptor [5], metabotropic glutamate receptor 2 [6], parathyroid hormone 1 receptor [7], bacteriorhodopsin [8-10] ), light harvesting complex II from spinach [11], bacterial tetrameric potassium channel [12], bacterial outer membrane protein A [13], Yersinia pestis outer membrane protein Ail [14], human transient receptor potential melastatin 4 [15] etc. Nanodiscs provide a stable and more biologically relevant bilayer environment for membrane proteins than typically used agents such as detergents or liposomes and have also been reported to improve protein stability and activity.
Nanodiscs, which are lipid-protein nanoparticles, can be found in nature in the form of HDLs or high-density lipoproteins, also known as good cholesterol [16]. These particles flow through blood transporting cholesterol to the liver for degradation. Initially, they are disc-shaped in the nascent form and assume a spherical shape with the intercalation of cholesteryl esters in the bilayer [17]. The lipid bilayer in HDLs is composed of a variety of phospholipids and cholesterol, and the surrounding scaffold protein is apolipoprotein A-1. What is known as nanodiscs today were first synthesized in the Sligar laboratory at the University of Illinois at Urbana-Champagne [18]. Typically, a single phospholipid or a mixture of two phospholipids is used to form the bilayer, and an engineered human apolipoprotein A-1 is used as the scaffold protein [19]. Nanodisc size depends on the length of the modified apolipoprotein A-1, also called Membrane Scaffold Protein or MSP. The zebrafish apolipoprotein A-1 has also been used to make nanodiscs [20], referred to as NABBs (Nanoscale apolipoprotein bound bilayers) [21]. Apolipoprotein A-1 directly purified from human serum has also been used to synthesize these reconstituted lipid discs [22, 23].
In addition to changing the scaffold protein, researchers have also varied the compositions of the phospholipid bilayer. From a single zwitterionic lipid to a mixture of zwitterionic and negatively charged lipids to bacterial total membrane lipids, the choice of the lipids generally depends on the protein at hand.
Incorporating integral membrane proteins into nanodiscs has many advantages over the more conventional methods such as solubilization in detergents micelles or liposomes.
Unlike detergents that form micelles around the protein, nanodiscs provide membrane proteins with a biologically relevant lipid bilayer environment. Nanodiscs are also more stable than liposomes. In addition, homogeneity in size is obtained relatively easily in nanodiscs than in liposomes, due to the fixed length of the MSP. Moreover, in nanodiscs, the percentage of boundary lipids (which are lipids in contact with protein and thus have different behavior from lipids in bulk), resembles more biological membranes than liposomes [24].
Experiments with rhodopsin (rhodopsin helps us see in dim light) show that when in nanodiscs the protein is as stable as when it is in the rod outer segment membrane of the retina [21].
P-glycoprotein 1 (an ABC-transporter implicated in multi-drug resistance in tumors) displayed greater activity in nanodiscs than in liposomes in the presence or absence of any substrate [25].
The number of membrane protein molecules in each nanodisc can be controlled by changing the lipid to membrane protein to MSP ratios. Effect of monomerization/dimerization on membrane protein function can thus be studied using nanodiscs. A recent publication has shown that even better control of oligomerization is possible when using covalently circular belt proteins instead of the usual linear ones [26].
Both ligand binding on the extracellular side and binding of signaling molecules on the cytoplasmic side can be studied in nanodisc incorporated proteins.
Ma Y et al, for example, reconstituted human apelin receptor in nanodiscs before immunizing a bactrian camel to obtain a single-domain antibody phage library [5].
Nanodiscs are synthesized by mixing together phospholipid/detergent micelles and MSP, followed by detergent removal. The size of the nanodisc is dependent on the length of the MSP protein, which is wound around the nanodisc like a belt. The ratio of phospholipids to MSP is a critical factor for successful nanodisc assembly and needs to be determined for each new combination of protein, phospholipid, and MSP. To incorporate a membrane protein into the disc, the protein solubilized in detergent micelles is also added to the nanodisc mix, before detergent removal (Figure 2). The ratio of phospholipids to MSP is a critical factor for successful nanodisc assembly and needs to be determined for each new combination of the integral membrane protein, phospholipid, and MSP.
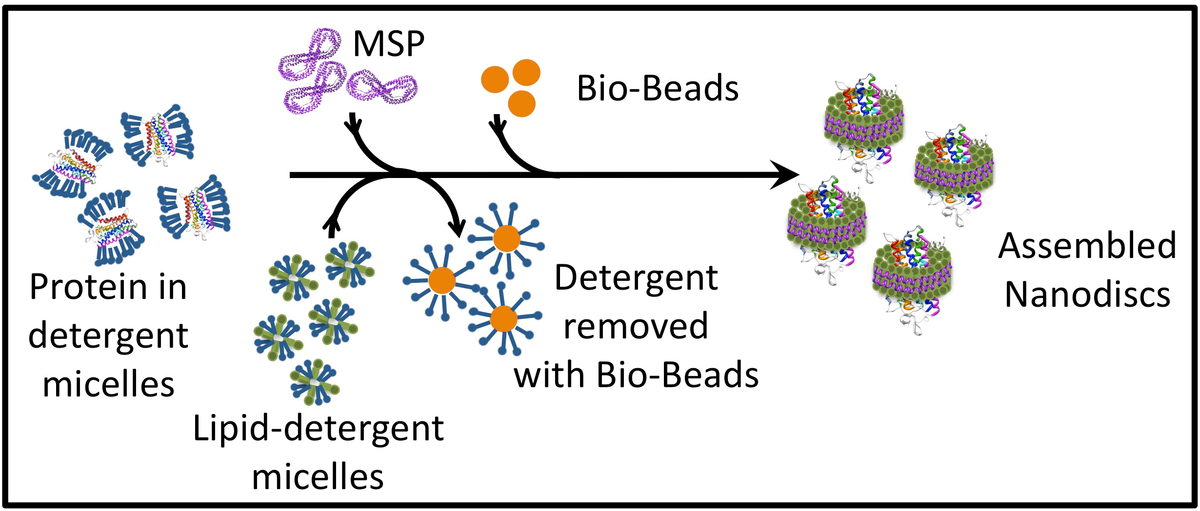
The membrane scaffold protein, which is a modified, recombinant, apolipoprotein A-1 from human blood, is expressed in bacteria. A variety of MSP clones coding for proteins of different lengths have been produced by the Sligar lab at UIUC [19]. MSP is produced in a soluble form in E. coli BL21Gold(DE3) and is purified by Ni2+ affinity chromatography following the protocols as described in [25] and [7]. The choice of MSP depends on the size of nanodisc required, i.e., incorporating a 7-transmembrane protein will need a larger nanodisc and consequently a longer MSP than a single-transmembrane protein. For example, MS Reid et al reported the structure of KCC4 in MSP1D1 nanodiscs determined by cryo-EM [27]. Zhou L and Sazanov LA reconstituted purified bacterial V/A-type ATP synthase from Thermus thermophilus in MSP1E3D1 and obtained its structure through cryo-EM [28]. MSP1D1E3 has been used to re-constitute nanodiscs with M2 muscarinic receptor [29] or P-glycoprotein [3]. The 6 or 7-His Ni2+ affinity tag can be removed before nanodisc assembly using TEV (Tobacco Etch Virus) protease. A recognition site for this protease exists between the protein and the His tag. Purification protocol for TEV has been described in [30]. Plasmids coding for MSP variants (like pMSP1, pMSP1E1, pMSP1E2, pMSP1E3, pMSP1D1, pMSP1E3D1, pMSP1E3D1_D73C, pMSP2, pMSP2N2) and TEV protease (pRK793) can be obtained from Addgene.
Most commonly used phospholipids in nanodiscs are zwitterionic (POPC [5, 27], DMPC, DPPC, etc.) or a mix of zwitterionic and negatively charged lipids (POPS [27], POPG [31], DOPG, etc.). Researchers generally mix zwitterionic and negatively charged lipids to mimic the cell membrane better. Bacterial membrane lipids have also been used for incorporation of bacterial proteins and others into nanodiscs [3, 32]. Nanodisc assembly occurs above the Tm of the lipid in use, where the lipids are in a liquid crystalline phase with fluid tails. Recent work has shown that having 25 mol% of a negatively charged lipid (DMPG) in a zwitterionic background (DMPC) drastically increases the stability of nanodiscs [Wadsäter M et al. The effect of using binary mixtures of zwitterionic and charged lipids on nanodisc formation and stability. Soft Matter, 2013,9, 2329-2337, doi:10.1039/C2SM27000E).
| Lipid | Tm(oC) |
|---|---|
| POPC(1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) | -2°C |
| POPS(1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine) | 14°C |
| POPG(1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) | -2°C |
| DPPC(1,2-dipalmitoyl-sn-glycero-3-phosphocholine) | 41°C |
| DMPC(1,2-dimyristoyl-sn-glycero-3-phosphocholine) | 23°C |
| DOPG(1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) | -18°C |
Detergent removal initiates spontaneous nanodisc assembly. Commonly used removal techniques include using beads like Bio-Beads™ and dialysis. If the detergent forms small-sized micelles, e.g., cholate, then dialysis is an option. If the detergent forms large micelles like n-Dodecyl-β-D-Maltoside, which cannot be dialyzed out, small hydrophobic Bio-Beads™ can be used to sequester the detergent from the mixture [3, 5]. These beads non-specifically adsorb small hydrophobic molecules, including detergents from the system. More information on the properties of Bio-Beads™, including their detergent binding capacity, can be found in [33].
| Detergent | CMC | Micellar Molecular Weight |
|---|---|---|
| SDS | 8 x 10-3 | 18000 |
| Cholate | 1.4 x 10-2 | 4300 |
| Deoxycholate | 5 x 10-3 | 4200 |
| C16TAB | 1 x 10-3 | 62000 |
| LysoPC | 7 x 10-6 | 92000 |
| CHAPS | 1.4 x 10-3 | 6150 |
| Octylglucoside | 2.3 x 10-2 | 8000 |
| n-Dodecyl-β-D-Maltoside (DDM) | 1.5 x 10-4 | 50000 |
| Digitonin | -- | 70000 |
| Triton X-100 | 3 x 10-4 | 90000 |
| Nonidet P-40 | 3 x 10-4 | 90000 |
| Tween 80 | 1.2 x 10-15 | 76000 |
Detailed protocols for incorporating a variety of different proteins into nanodiscs can be found in the literature. A very useful starting point that lists different considerations while making nanodiscs is described in depth in [25]. A detailed protocol for bacterial chemoreceptor incorporation into nanodiscs is described in [34]. More information on protocols for nanodisc preparation can also be found on the Sligar laboratory website. Furthermore, a number of reagents required for making nanodiscs can be obtained from Sigma-Aldrich or Cube Biotech. An excellent source for phospholipids is Avanti Polar Lipids.
A recent publication describes a new circularized belt protein that forms nanodiscs called circular nanodiscs (cNDs). The N and C termini of the belt protein are covalently linked to each other before nanodisc formation [26]. As in linear nanodiscs, the size of the circular belt protein is chosen based on the predetermined size of the circular nanodisc. These nanodiscs are prepared in the standard way as described elsewhere in this article. The lipids in cNDs are observed to be more restricted and the nanodiscs themselves more stable and homogenous in size compared to nanodiscs with the classical linear belt protein. cNDs were employed to trap the channel VDAC-1(human voltage-dependent anion channel-1) and measure its 2D 1H-15N TROSY HSQC spectra. The spectra obtained were dramatically better than those obtained in linear nanodiscs. The spectra also allowed distinction between monomers and dimers of VDAC-1 which were encircled in circular belt proteins of different lengths. cNDs were better at controlling the oligomerization state of proteins than traditional nanodiscs.
Membrane proteins have been incorporated into nanodiscs for a variety of different studies. A list of some of these studies is given below. For an in-depth review of the various ways that nanodiscs have improved our understanding of membrane proteins and membrane-associated phenomenon, please see reference [35].
GPCRs are the largest gene family in the human genome and are targets of about 40% of all pharmaceutical drugs. How GPCRs function, is thus an important area of study. Multiple GPCRs have been incorporated into nanodiscs to study their ligand binding and G protein interactions. Since the number of GPCR monomers per nanodisc can be controlled, these studies have provided useful information on the effect of dimerization on GPCR activity. Studies on multiple GPCRs, e.g., rhodopsin, beta2-adrenergic receptor, neurotensin, opioid and metabotropic glutamate receptor 2 have all clearly shown the sufficiency of a monomer for G protein activation. Using nanodiscs, it has been demonstrated that monomeric rhodopsin can undergo phosphorylation and bind arrestin.
Unlike studies in cells or cell membranes, nanodiscs allow much more precise control of experimental conditions, e.g., it is easier to specify the concentrations of buffer, salt, and any interacting proteins. In a recent study, this precise control resulted in the discovery of novel effects of Ca2+ on ligand binding and G protein activation in the Family B GPCR parathyroid hormone 1 receptor [7].
Accurately controlling the lipid composition of nanodiscs has resulted in unprecedented insights into the effects of lipids on the function of a variety of membrane proteins. It has been observed that the composition of lipids in the nanodisc bilayer can be directly correlated with the efficiency of G protein/arrestin association with the activated GPCR; increasing negatively charged lipids like POPS or POPG in a background of POPC increased this association [36, 37]. Such modulation of interaction between proteins has also been observed in other systems. For example, the interaction between the blood clotting factor X and factor VIIa·tissue factor complex is affected by the mol% of POPS in the POPC environment of the nanodisc [38]. The glycosylation of membrane-embedded oligosaccharyltransferase complexes was assessed through MSPE3D1 nanodiscs [39].
Encasing membrane proteins in nanodiscs are a preferred way to fish for their binding partners. Usual methods involving solubilizing the protein in detergent can potentially disrupt biologically relevant protein-protein interactions. Using liposomes are also not ideal. They are heterogeneous in size and also lead to non-specific hydrophobic interactions. The usefulness of nanodiscs in finding binding partners was recently demonstrated in a SILAC-based mass spectrometric study [40]. The authors were able to identify binding partners that are known to be susceptible to even the mildest detergent. Interestingly, the lipid composition of the nanodisc affected the capture of binding proteins.
Commonly used detergent-based methods applied for the purification of membrane proteins often leads to the disruption of their structure. One approach to minimize the effect of detergent on a membrane protein is to reduce the duration of contact of the protein with the detergent. In a recent study, authors have used the family B GPCR parathyroid hormone 1 receptor to demonstrate a nanodisc-based purification protocol [7]. The authors solubilized cells expressing the receptor in detergent and immediately proceeded to incorporate the total membrane protein into nanodiscs. Following this, an affinity purification step yielded the desired protein in nanodiscs. This protocol can be extended to other family B GPCRs and quite likely other membrane proteins.
Current vaccines against influenza virus usually involve using either inactivated or live attenuated viruses, produced in chicken eggs. It has been shown that nanodisc-incorporated influenza virus hemagglutinin elicits an immune response similar to the commercial vaccines available now [41]. The recombinant hemagglutinin-nanodisc is much easier to produce than whole viruses and does not contain contaminating chicken proteins.
A variety of techniques that have been developed for soluble proteins can now be more easily applied to membrane proteins in nanodiscs, which provide a homogenous solution-based environment for these proteins. Apart from the more conventional methodologies that are employed to study proteins, methods like NMR and surface plasmon resonance are being used to study membrane proteins in nanodiscs.
Nanodiscs have been used for 2D heteronuclear NMR experiments on membrane proteins such as the membrane-spanning fragment of human CD4 [42], human voltage-dependent anion channel (VDAC-1) [43], VDAC-2 [44], the voltage-sensing domain of the archaeal potassium channel KvAP [45] and the alpha-helical dimeric protein YgaP from E. coli [46]. Recently, nanodiscs have been optimized for more complicated solution NMR experiments by the Wagner group at Harvard Medical School [8]. The authors truncated the MSP to facilitate the formation of smaller nanodiscs ranging in size from 6.5 to 9.5 nm, with less overall lipids and consequently lower molecular weight than the more conventional nanodiscs. The authors used the eight-stranded antiparallel beta-barrel protein, OmpX, as a model system to show that along with its small nanodisc size, high deuteration levels of proteins and lipids and state-of-the-art non-uniform NMR sampling methods enable high-resolution structure determination. Recently the same group has shown that using a circular instead of a linear belt protein allowed better NMR signals the channel protein VDAC-1 [26].
In-vitro translation methods can result in a newly formed membrane protein getting co-translationally incorporated into a nanodisc. A recent paper has shown that these nanodisc incorporated integral membrane proteins can then be moved into isotropic bicelles, with detergent titration, that results in much better signals [47]. This method prevents membrane proteins from ever being exposed to detergents during purification/extraction and is useful for those membrane proteins that cannot tolerate dissociation from their lipid environments as is necessary during standard membrane protein purification whether in detergent micelles or in nanodiscs.
Although SPR has most often been used to study soluble protein-ligand interactions, recently membrane proteins in liposomes have been used as well. Substituting the liposome platform with nanodiscs has many advantages. The most obvious one is directionality. In liposome preparations, not all protein molecules will be oriented the same way. Thus the ligand will sometimes “see” the non-ligand-binding side. In nanodiscs, the same side of the protein, either extracellular or intracellular (depending on experiment design) will face the ligand. If the MSP is used to tether the discs, both sides of the protein will be accessible. If a tag on the protein itself is used, then the non-tagged side will be accessible. Moreover, nanodisc preps are more stable and uniform. Examples of studies using nanodiscs-incorporated proteins in SPR can be found in [38, 48] and [49].
Nanodiscs are ideally suited for single molecule studies. Cytochrome P450 isoform 3A4 (CYP3A4) is the principle human drug-metabolizing enzyme. A study by TIRFM (Total internal reflection fluorescence microscopy) on nanodisc-incorporated CYP3A4 gave direct evidence that effectors like alpha-naphthoflavone enhanced substrate metabolism of the enzyme by affecting the residence time of the substrate [50].
Another single-molecule method, SMFS (Single molecule force spectroscopy), has been applied to bacteriorhodopsin in nanodiscs [9]. The study showed that there is no distinguishable difference in the structure, unfolding intermediates and inter- and intramolecular forces in bacteriorhodopsin in nanodiscs and in native purple membranes. Additional details of methods used to study nanodiscs at the level of a single molecule can be found in [51].
Cryo-electron microscopy has been employed to study to various nanodisc enclosed membrane proteins at high resolution. An example is the TRPV-1, transient receptor potential cation channel subfamily V member 1 an ion channel protein. Lipids surrounding the protein are shown to interact with the ion channel thereby affecting its interaction with ligands [52]. Other proteins studied this way are a bacterial V/A-type ATP synthase from Thermus thermophilus [28], another transient receptor potential ion channel family protein, PDK2 [53], the Tc toxin, TcdA1 from Photorhabdus luminescens [54], yeast oligosaccharyltransferase with MSPE3D1 [39], Gram-negative bacteria, ATP-binding cassette transporters LptB2FG and LptB2FGC [31], and DCPIB-inhibited mouse volume-regulated anion channel LRRC8A [55].
Increased homogeneity is one of the characteristics of nanodiscs-based structures which significantly improve the quality of electron microscopy. In particular, conformational analysis of integrins was performed using nanotubes [56]. In addition, the advantages of nanodiscs include protection from aggregate formation and conservation of the molecular structure of the studied proteins as reported for ryanodine receptor [57] and TRP1 channel [52]. Combination of nanodiscs and EM was proved to be very effective for the analysis of structure and protein-protein interactions in various signal transduction pathways [58, 59].
Additional details of methods used to study nanodiscs at the level of a single molecule can be found in [51].
Since the stability of protein molecules assembled into nanodiscs is high, nanodiscs may be used for X-ray crystallography and other methods. According to several studies, proteins would be assembled and preserved in nanodiscs [60, 61]. To optimize the analysis conditions for direct crystallization and improve structural analysis by X-ray, peptide molecules are attached to each other to form a two-dimensional network [62].
The nanodisc technology makes it easier to control the structure and interactions of membrane proteins in a membrane region. For instance, a combination of mass spectrometry-based proteomics and nanodiscs was applied to study interactions between surface proteins [40]. Besides, nanodisc-based methods were used to investigate between lipids and membrane proteins. For example, nanodiscs with inserted ganglioside were applied to analyze the binding targets of cholera toxin [63].
Analysis of surface proteins inserted in nanodiscs may also be performed using neutron scattering. In particular, studies of conformation and other structural characteristics of the membrane lipid layer were performed by X-ray scattering [64]. Also, neutron reflectometry was applied to detect structural variations of surface protein molecules in nanodiscs, such as modifications of rhodopsins upon physiological activation [65].
The key factor in making reproducible, stable nanodiscs is the molar ratio of the membrane scaffold protein to lipids. The length of the MSP changes the size of the nanodisc and thus affects this ratio. Additionally, a change in the lipid composition will also affect this ratio because of the area occupied by the polar head groups of lipids changes with each lipid. For example, a nanodisc with the scaffold protein MSP1D1 holds ~ 65 POPC and ~ 90 DPPC molecules, while the scaffold protein MSP1E3D1 produces nanodiscs that can hold ~ 130 POPC and ~ 180 DPPC molecules per leaflet, respectively [25]. Moreover, when there is a target integral membrane protein incorporated in the nanodisc, the lipid molecules that are displaced by the protein also need to be considered. Analysis of purified nanodiscs has shown that rhodopsin displaces ~ 50 POPC molecules [66]. Thus, for each new combination of MSP, lipid and target protein, different MSP to lipid ratios have to be tested. Initial analysis of the nanodisc is usually done using size exclusion chromatography where the presence of a homogenous species in the expected size range is tested. Alam A et al reconstituted ABCB1 nanodiscs with MSP1D1 and a brain polar lipid (BPL) and cholesterol mixture (80:20, w:w) at a 1:10:350 (ABCB1:MSP1D1:Lipid) molar ratio for 30 minutes atroom temperature and completed the reconstitution with the addition of 0.8 mg/ml pre-washed Bio-rad Biobeads for 2 hours at room temperature [4].
It is known that certain membrane proteins when exposed to detergents permanently lose their activity. With styrene-maleic acid copolymers (SMA) it is possible to directly form nanodisc shaped particles from native bilayers without resorting to detergents [67-69]. The hydrophobic groups in SMA insert into the lipid bilayer, solubilizing it while forming nanodiscs at the same time. These nanodiscs have natural membrane lipids surrounding the protein. The SMA polymers form a belt-like structure around the nanodisc thus protecting the lipid bilayer from the surrounding aqueous layer. These nanodiscs have been referred to as ‘native nanodiscs’ due to the native lipid bilayer around the membrane protein. The method has opened up the possibility of extracting membrane proteins from cells without ever separating them from their immediate membrane environment. Proteins including bacteriorhodopsin and mitochondrial membrane complexes have been studied this way [70, 71]. An E. coli potassium channel KcsA has been recently purified in native nanodiscs using Nickel affinity chromatography [12]. The SMA polymers have also been modified with sulfhydryl groups for easy tagging of nanodiscs with desired thiol-reactive groups [72] or modified, for example, SMA-QA, to achieve ultra‐stability towards divalent metal ion concentration over a wide pH range [73, 74].
This article briefly describes recent advances in nanodisc technology. For more details on various aspects of nanodiscs assembly, applications and function, please see [2, 75] and [76].
- Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553-82 pubmed
- Bayburt T, Sligar S. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci. 2003;12:2476-81 pubmed
- Denisov I, Grinkova Y, Lazarides A, Sligar S. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477-87 pubmed
- Whorton M, Jastrzebska B, Park P, Fotiadis D, Engel A, Palczewski K, et al. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387-94 pubmed
- Whorton M, Bokoch M, Rasmussen S, Huang B, Zare R, Kobilka B, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682-7 pubmed
- Denisov I, McLean M, Shaw A, Grinkova Y, Sligar S. Thermotropic phase transition in soluble nanoscale lipid bilayers. J Phys Chem B. 2005;109:15580-8 pubmed
- Boldog T, Grimme S, Li M, Sligar S, Hazelbauer G. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc Natl Acad Sci U S A. 2006;103:11509-14 pubmed
- Rigaud J, Pitard B, Levy D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta. 1995;1231:223-46 pubmed
- Boldog T, Li M, Hazelbauer G. Using Nanodiscs to create water-soluble transmembrane chemoreceptors inserted in lipid bilayers. Methods Enzymol. 2007;423:317-35 pubmed
- Shaw A, Pureza V, Sligar S, Morrissey J. The local phospholipid environment modulates the activation of blood clotting. J Biol Chem. 2007;282:6556-63 pubmed
- DeVree B, Mahoney J, Velez Ruiz G, Rasmussen S, Kuszak A, Edwald E, et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535:182-6 pubmed
- Bayburt T, Leitz A, Xie G, Oprian D, Sligar S. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875-81 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
