Immunoglobulins (Ig) or antibodies are an important component of the immune system where they carry out a dual role which is intrinsic in their structure. They can 1) bind specifically target antigens (Ags) through the fragment antigen binding (Fab) site and 2) induce an immune response by activating other cells of the immune system through the Fc region (Figure 1). They are classified in polyclonal - different antibodies that recognize different epitopes on the Ag, and monoclonal antibodies (mAbs). The latter are highly specific antibodies all recognizing one epitope on the Ag and they were first described nearly 40 years ago. During the years mAbs have become an invaluable tool in basic laboratory research since they are extensively used in immunohistochemistry, flow cytometry, western blot and related techniques [1]. Besides this use, in the last 20 years mAbs have been an important component of cancer therapy. Their therapeutic utility has been extended to chronic inflammatory disease, transplantation and infection (i.e., mAbs anti-HIV have shown promising results in human HIV therapy). The interest in this class of Abs has increased over the years as well as the research in this area.

The human antibody molecule consists of two identical heavy (H) chains and two identical light (L) chains, covalently linked by disulphide bonds. Each chain contains a variable domain (VH and VL) for Ag recognition and a constant region for the effector function (CH1-3 and CL) (Figure 1). The H chain is also composed by a hinge region. Based on the Fc portion, five classes of antibodies can be distinguished: IgG, IgM, IgD, IgA, and IgE. Both H and L chains are characterised by variable regions of 110 amino acids at the amino terminus which contain three hypervariable regions called complementarity determining regions (CDR1, CDR2 and CDR3) which are embedded into four conserved framework regions (FRs). The CDR3 is normally the most variable regions and the center of the Ag binding site.
Human mAbs can be produced from hybridoma cell lines, display technologies, such as phage display library, and single-sorted human B cells. Several other methods more advanced and which will not be exploited here in details include [2] :
- Engraftment of a complementary-determining region (CDR) from a mouse hybridoma cell line into the human variable light and heavy framework regions;
- Use of transgenic mice which express DNA encoding human Ig genes which is based on the traditional hybridoma method to express the human mAbs.
The hybridoma technique is one of the oldest methods and also the most popular for generating mAbs. Many laboratories are still using this method which is based on the fusion (hybridoma) of antibody-producing B cells with an immortal myeloma cell line in a selective medium where only the hybridoma cells can survive producing a specific mAb. For example, Zhao Y et al immunized mice with lipid A-containing liposomes mixed with antigen proteins to generate monoclonal antibodies against AMPA receptors [3].
B cells are extracted from a spleen of a mouse previously immunised with the specific antigens towards which the mAbs are generated bringing to the enrichment of an antigen-specific B cell population. The B cells extracted are then mixed (i.e., fused by chemical or virus-induced techniques) with a selected myeloma B cell line which lacks the gene hypoxanthine-guanine-phosphoribosyltransferase (HGPRT) and is not able to produce antibodies itself. This selection is important since these two B cell populations are then cultured in a selective medium containing hypoxanthine-aminopterinthymidine (HAT medium) where only the hybridomas can survive since the myeloma cells are not able to divide in the HAT medium and the specific antibody-producing B cell will die after several rounds of division. Therefore, only the hybridoma cell line can both divide and proliferate. After screening for selecting the hybridoma clones that produce the mAbs of interest, these mAbs are then purified from the culture supernatant. Although this method is still the gold standard for mAbs production, it has some limitations i.e., the efficiency of immortalization and fusion are often low or the importance of the maturation status of the B cells [4]. In order to overcome this limitation, in 2003 the laboratory of Michel Nussenzweig developed an efficient method to clone and expressed mAbs starting from single FACS-sorted B cells.
An efficient way to produce human mAbs is based on single cell sorting of B cells by flow cytometry, amplification of the immunoglobulin (Ig) genes (both H and L chain genes) by PCR, and subsequent Ig gene expression vector cloning for antibody production in vitro.
The strategy of the single B cell sorting to produce human mAbs is based on the amplification of the human IgH, Igκ and Igλ genes by PCRs from single cell cDNA. PCRs are performed using specific forward and reverse primers to amplify the heavy and light (κ and λ) chain separately. After the amplification the PCR products are cloned into a specific expression vector to allow the expression of the heavy and light chain. The vectors are then used to transfect human cell line in order to express human mAbs which are then purified from the culture supernatant. A detailed protocol can be found in [4]. Quite a few antibodies against SARS-CoV 2 have been reported through this approach, in response to COVID-19 pandemic [5, 6].
Phage display was introduced almost 30 years ago and it was originally developed by Greg Winter et al and Richard Lerner, Carlos Barbas et al at the MRC Laboratory of Molecular Biology and the Scripps Research Institute, respectively. Antibodies produced in the phage display libraries can be expressed either as Fabs, scFvs (single-chain variable fragments) or sdAbs (single-domain antibodies) (Figure 1 and 2a). The antibody sequence is normally fused with the gene III coat protein [7-9].
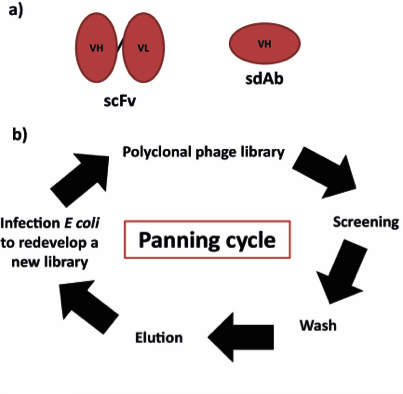
The principle of all antibody phage display libraries is based on the physical link of antibody specificity and affinity (phenotype) with the sequence of the phage particle (genotype). This step is then followed by a rapid in vitro selection of antigen-specific antibodies. As summarised in Figure 2b, polyclonal phage expressing recombinant antibodies on their surface are screened towards target antigens immobilised on magnetic beads, polystyrene surfaces or on the surface of whole cells. After several stringent washing to get rid of the unbound phage, antigen-bound phage is eluted through a change in pH or digestion with proteases. The selected phage is then used to infect again E. coli which will produce a new library for another cycle of screening. This “panning cycle” is done until the library is enriched in the monoclonal phage. The antibody sequence is recovered and used to express the recombinant antibody [8].
The main source for antibody sequences is represented by The ImmunoGenetics Database (IMGT) (http://www.imgt.org/). IMGT is the global resource for immunoglobulins which is updated regularly and it contains all the germline gene sequences of the antibodies. A second useful website for antibody sequence is IgBLAST (http://www.ncbi.nlm.nih.gov/igblast/) which has been developed at NCBI specifically for immunoglobulin variable domain sequences. For antibodies structure data, some resources are represented by Protein Data Bank (PDB) (http://www.rcsb.org/pdb/home/home.do), CATH database website (http://www.cathdb.info), antibody crystal structure (SACS), IMGT-3D structure-DB.
There are several useful tools when working with antibodies sequences. Among all, for antibody modelling, Rosetta Online Server (http://rosie.rosettacommons.org) is one of the useful open access websites for 3D structure prediction. For glycosylation prediction, NetNGlyc1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc) is used to predict in human antibodies the presence of N-glycosylation sites; NetOGlyc4.0 (http://www.cbs.dtu.dk/services/NetOGlyc) Server to predict O-glycosylation sites.
Multiple expression systems, vectors, and transfection reagents are available to prepare different formats of antibodies in vitro. 293 or Expi293F cells appear to be a popular choice [10, 11]. Rigau M et al expressed BTN2A1 antibody clones in Expi293F cells [12]. Tao Y et al prepared bispecific diabodies and other formats of antibodies using Expi293F cells from Thermo Fisher, FectoPRO transfection reagent from Polyplus-transfection, and rProtein A sepharose from GE Healthcare [13].
The last step in antibody production is their expression in a host system. Choosing which cell line to transfect is critical and must be evaluated before the initiation of any development expression program. Indeed, changing the expression system later could affect the expected activity of the antibody which you are producing.
The importance of selecting the host system is related mainly to the post-translational modification introduced into the protein. In particular, for antibodies, glycosylation is one of the most common post-translational modification that can affect folding, stability, solubility, protein activity, immunogenicity, etc. [14].
Antibodies produced in cell-free systems are difficult to fold properly. Glycosylation is missing in E. coli expression system.
The expression systems include bacterial, yeast [15], mammalian, insect, plant as well as in vitro systems. Frenzel A et al reviewed comprehensively various hosts, production systems, and yeilds, with the highest yield achieved in CHO and HEK293 cell lines [16]. Normally, for monoclonal antibodies production, mammalian cells are used for their ability to correctly assemble and introduce post-translational modifications (Handbook of Therapeutic Antibodies 2E (2014)). Several mammalian cells can be used as host systems but the pattern of glycosylation has been showed to be unique for each system. Normally, in a research laboratory the cell lines successfully used to express antibodies are the Chinese hamster ovary (CHO) cells or the human embryonic kidney (HEK) cells (HEK293). However, CHO-based transient transfection is likely to be characterised by poor transfection efficiency with a subsequent low amount of protein produced. Therefore, HEK293 is the main cell line used for transient transfection. Independently of which cell line you will choose, it is important to bear in mind that the pattern of glycosylation of the Ab produced will always be different from the real circulating in vivo pattern of glycosylation. CHO cells are commonly used for large-scale production of antibodies. HEK293 cells tend to clump and need special treatment for large-scale production. For example, Zhao Y et al used the pET-22b vector with a pelB signal peptide and a C-terminal Strep II tag to express the 11B8 scFv construct against GluA1 receptor in Escherichia coli BL21 cells and a biscistronic pFastBac1 vector with the Fab domains of the GluA2-specific 15F1 antibody heavy chain and light chain, including a Strep tag at the C-terminus of the light chain and GP64 signal peptides (MVSAIVLYVLLAAAAHSAFA) at the N-terminus of the heavy and light chains, to produce the 15F1 Fab fragment in baculovirus- infected Sf9 cells [3]. Choi J et al expressed chimeric antibodies FreeStyle 293-F serum-free and suspension-adapted HEK293F cells and purified them by affinity chromatography with Protein L and Protein A columns [17]. T Hettmann et al expressed humanized antibodies against the modified beta amyloid peptide in Freestyle™ CHO-S cells from Thermo Fisher Scientific [18].
The table below summarises the main types of HEK cells that can be used for transient transfection.
| Type | Characteristic | |
|---|---|---|
| 293 | Derived from human embryonic kidney cells. | Adherent cells |
| 293T | Parental line for 293. The T means that it expresses the Large T antigen important for the replication of plasmids that contain the SV40 replication origin. | Adherent cells |
| 293FT | Fast growing variant of the 293T cell line. | Adherent cells |
| Expi293F / FreeStyle 293F | Derived from 293 cell line. Grow at high density. They are highly transfectable and generate superior protein yields compared to 293 and 293T cell line. | Suspension cells |
The world of commercially available transfection reagents kits is very wide. Therefore, it is important choosing the best one accordingly to your experiment. The first question to ask regards the yield you would like to obtain which is related on the type and number of tests you need to perform with the Abs produced.
The most common and less expensive transfection reagent is the polyethylenimine (PEI). PEI is a cationic polymer able to condense the DNA into positively charged particles which are brought into the cells via endocytosis. Once inside the cell, the vesicle releases the polymer-DNA complex into the cytoplasm. If the complex unpacks then the DNA is able to diffuse into the nucleus. The main limit of PEI-based transfection is the low yield obtained which is in the range of micrograms. However, it is good enough in the first screening of a library of Abs. For example, Maun HR et al transfected pRK mammalian cell expression vectors into mammalian cells (CHO, CHO DKO, HEK293 or HEK293 DKO) with 25 kDa linear PEI from MilliporeSigma [19].
Nowadays several transfection reagents that allow a better yield have been introduced in the market. Although more expensive, they are more efficient. A good list of the main transfection reagents can be found on the Thermo Fisher Scientific website (http://www.thermofisher.com). A summary table is reported below.
| Reagent | Adherent cells | Suspension cells | Comments |
|---|---|---|---|
| Lipofectamine | Good efficiency | Low/medium efficiency | There are several types of lipofectamine reagents. For more details visit http://www.thermofisher.com |
| ExpiFectamine [20] | No | Used for high-density suspension cell culture; it uses enhancers that boost transfection performance and protein expression | Milligrams to grams of protein yield |
| FreeStyle MAX | No | Optimized for CHO suspension cells | Milligrams protein yield |
| JetPrime | Good efficiency | No | Milligrams protein yield |
The expression vectors are also other important components in the expression systems and they are now well established. The optimization of the expression vectors is mainly focussed on the identification and optimization of strong promoters and enhancer in order to increase the level of transcription and translation. In particular, specific promoter/enhancers are used such as the cytomegalovirus (CMV) [20], SV40, elongation factor promoter, polyoma enhancer, chicken beta-actin promoter. For antibody production, the transient transfection is normally performed with two distinct vectors, one encoding the H chain and one encoding the L chain. However, co-transfection has some limitations such as the need for a large amount of vectors which affects the cost and it is labor- and time-consuming. There are some studies trying to overcome this limitation by introducing vectors that can express the H and L chain simultaneously without the need for two vectors (one-step assembly) [21].
Most of the antibodies used in biotherapeutics are humanised or chimeric mAbs. Although mAbs have been proved to be very successful, nowadays new non-canonical form of antibodies has been introduced, in order to improve tissue penetration, pharmacokinetics, and effector function [22]. New classes of antibodies include Fab fragments (L chain and VH-CH1 domains linked by a disulfide bond) [20], Fv fragments (V domains), single-chain antibodies-scFvs (V domains linked by a flexible peptide) (for example, clone 4A6 scFv against myc tag [23] ). There are many other forms of antibody fragments. For instance, two antibody fragments with different specificities can be fused to produce bispecific or diabodies. A depth description of the new form of non-canonical antibody and their application can be found at here.
Normally, the antibody produced are of the IgG isotype. Therefore, the method of choice for their purification is based on affinity chromatography with protein A or G. A summary table for human antibodies is reported below:
| Species | Subclasses | Protein A binding | Protein G binding |
|---|---|---|---|
| Human | IgA | variable | - |
| IgD | - | - | |
| IgE | |||
| IgG1 | ++++ | ++++ | |
| IgG2 | ++++ | ++++ | |
| IgG3 | - | ++++ | |
| IgG4 | ++++ | ++++ | |
| IgM | variable | - |
Purification of antibodies via affinity chromatography is based on a reversible interaction between the Fc portion of the antibody and a specific ligand (i.e., protein A/G) coupled to a chromatography matrix. Protein A and G have a bacterial origin, from Staphylococcus aureus and Streptococcus, respectively. Resins made with Protein L, which binds the V kappa light chain variable region has also been used [17]. After the binding with the ligand (binding phase), the interaction is normally reversed by changing the pH during the elution phase. Affinity chromatography is an efficient one-step system to purify Abs starting from a large volume (e.g., culture supernatant) with a good degree of yield recovery. Protein A or G can also be used to purify heavy chain heterodimers for bispecific antibodies if protein A or G binding in one of the heavy chains is removed [24]. MabSelect Sure resin from GE Healthcare, a protein A matrix, appear to be a good choice. Rigau M et al purified BTN2A1 antibodies with MabSelect Sure resin [12]. Maun HR et al purified human IgG4 antibodies with MabSelect Sure resin, then with a Superdex S200 10/300 GL size exclusion column from GE Healthcare [19]. Inoue K et al first salted out 2C10 hybridoma supernatant with half-saturated ammonium sulfate, and then purified the antibody, an IgG2b with kappa light chains, with the Protein G HP Spin Trap from GE Healthcare [25]. Thermo Fisher CaptureSelect CH1-XL Affinity Matrix binds to the CH1 domain of all subclasses (1, 2, 3, and 4) of human IgG and Fab fragments regardless of the light-chain isotype [20].
The Antibody Purification Handbook from GE Healthcare website is very useful since it contains the most used strategies for sample preparation and purification of antibodies and it can be used as a reference point when you are planning to perform purification of your antibodies [26].
Purification at small scale is very useful in antibody screening experiments. It can be achieved by i) using chromatography columns (i.e., disposable polypropylene spin columns) that can be packed or that can be already pre-packed with protein A or G; ii) 96-well plates already pre-packed with protein A or G for high-throughput screening. In the second system the antibodies are loaded into the wells and both washing and elution can be done by centrifugation or by using a vacuum system.
Occasionally at a laboratory scale, thus not for the industrial purpose, it is necessary to produce antibodies in different quantities, from microgram to gram scale. Therefore, using an automated purification system which will increase the yield of both quantity and quality is very important. On the market, there are several automated purification systems that use the ÄKTA chromatography machine mostly from GE Healthcare. These systems give more reproducible results and are very useful when the manual purification (see above – small scale purification) becomes time-consuming for different reasons: i) the need to obtain more antibodies, ii) the starting sample volume is large, iii) many samples to be purified. An automated chromatography system can be quite expensive but there are several applications where investing in these systems can save time, effort and sample and most important bring to a better yield. GE Healthcare has several ÄKTA machines. The main ones are listed below:
- 1. ÄKTA start
- 2. ÄKTA prime plus
- 3. ÄKTA xpress
- 4. ÄKTA pure
- 5. ÄKTA avant
- 6. ÄKTA pilot
- 7. ÄKTA ready
- 8. ÄKTA process
The ÄKTA machines from 1 to 5 are for a laboratory scale. The ones from 6 to 8 are for manufacturing and production (industrial level). More details can be found at the GE Life Sciences website [26].
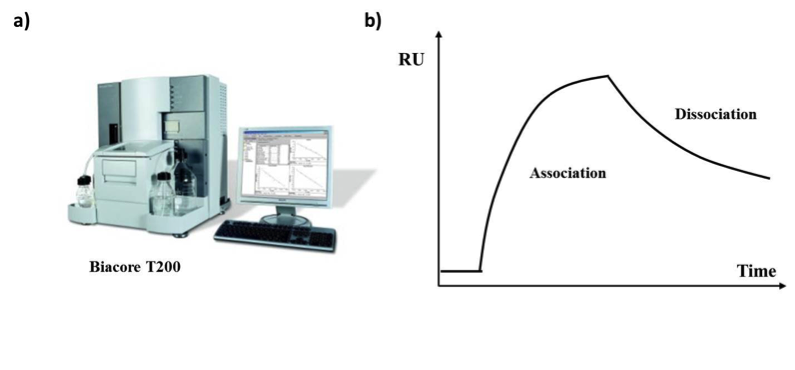
Biacore systems (Figure 3a) use the principle of surface plasmon resonance (SPR) to measure the interaction between molecules in real-time. The analysis involves two interacting molecules, one is attached to the surface of a sensor chip (ligand) and one is found in solution (analyte) passing over the ligand. The antibody can be either the ligand or the analyte. After the ligand binds the analyte the sensor surface generates a response proportional to the bound mass and this is used to determine the concentration in the picomolar and nanomolar range. The Biacore system is very useful when studying antibodies since it can be used to address:
- The specificity of interaction between the antibody and the antigen;
- The kinetics and affinity of an interaction between the antibody and the antigen;
- The concentration of specific molecules.
Changes in the resonance signal are expressed in resonance units (RU) and they are usually plotted against time and are presented as a Sensogram (Figure 3b). Antibody-antigen affinity can also be measured by Bio-layer interferometry (BLI) [15], ELISA, and microscale thermophoresis (Nano-differential scanning fluorimetry /nanoDSF) such as NanoTemper [17, 27].
D Wrapp et al captured His-tagged SARS VHH or MERS VHH with the NTA sensorchip and measured their bindings with SARS-CoV-1, SARS-CoV-2 or MERS-CoV S protein fragments using a Biacore X100 from GE Healthcare [15]. Tao Y et al measured the antibody-protein binding kinetics using BLI assays with an Octet HTX instrument from ForteBio and using SPR assays with a ProteOn XPR36 system from BioRad [13]. Apparent affinities between an antibody and its cognate antigen expressed on the surface of cells such as CHO can also be determined through flow cytometry [28].
Nowadays, the production of monoclonal antibodies is not only limited to laboratory use but there are several antibodies which are produced for the therapeutic purpose. Therefore, the standard procedures applied in a standard laboratory can be applied mostly for screening than for therapeutic use. Indeed, generating antibodies in large quantities to be used in humans requires more refined steps and controlled procedures which should be standardised in order to generate a platform for high titer antibodies production. In particular, it involves i) set up a stable transfection rather than a transient transfection, ii) using bioreactors to grow cells, iii) remove cells from the secreted product and purify the antibody from the cell culture supernatant with several chromatography and filtration steps, iv) change the buffer and resuspend the antibody in the desired formula for clinical use. All these steps require continuously quality control tests to ensure the safety and biological activity. Therefore, all these steps can be supported only at the industrial level more than in a standard research laboratory.
- Kodoyianni VMH, Curet M, Kravitz R, and Schram B. A Novel Process for Developing Fully Human Monoclonal Antibodies. Pharmaceutical Technology. 2012.
- Hoogenboom H. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105-16 pubmed
- Steger K, Brady J, Duskin M, and Donato K. Literature Review: CHO versus HEK Cell Glycosylation. Available from: www.maxcyte.com/
- Antibody purification. Available from: www.gelifesciences.com/solutions/protein-research/knowledge-center/protein-purification-methods/antibody-purification
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Antibody Applications
- Antibody Companies
- Antibody Dilution and Antibody Titer
- Antibody Quality
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- Aptamers and Affimers
- Beta Actin Antibody
- Bispecific Antibodies and Trispecific Antibodies
- BrdU Antibody, DNA Antibody, RNA Antibody and Hybrid DNA:RNA Antibody
- GFP Antibody
- HA Hemagglutinin Tag Antibody and FAQs
- Mouse Antibody
- Multiplexing Immunohistochemistry
- Myc Antibody Review
- Phage-Display Technology for the Production of Recombinant Monoclonal Antibodies
- Phosphotyrosine Antibody
- Protein Purification
- Rat Antibody
- Secondary Antibodies
