One of the dilemmas of protein characterization in biological samples is the dominance of high abundance proteins which complicate the detection and analysis of low abundance proteins (LAPs). In the case of blood plasma, a total of 22 proteins, albumin, total IgG, transferrin, fibrinogen, IgA, alpha-2-macroglobulin, IgM, alpha-1-anti-trypsin, C3 complement, haptoglobulin, alpha-1-acid glycoprotein, apolipoprotein-B, apolipoprotein-A1, lipoprotein a, factor H, ceruloplasmin, C4 complement, complement factor B, pre-albumin, C9 complement, C1q complement, and C8 complement, comprise 99% of the total human plasma protein content, while hundreds of other proteins contribute to the remaining 1% [3, 4]. Recent studies have demonstrated that characterization of LAPs can be performed by biochemical and molecular biology methods, such as mass spectrometry (MS), Western blotting (WB), nuclear magnetic resonance (NMR), or single-molecule peptide sequencing [5, 6], either with the presence of high abundance proteins or with the enrichment of LAPs.
Several methods for LAP enrichment have recently been developed (Table 1). A novel approach based on metabolic glycoprotein labelling and click chemistry for the enrichment of glycoproteins, named "improved Secretome Protein Enrichment with Click Sugars" - iSPECS, has been presented during the SFN 2018 meeting [7]. Blood proteins can also be enriched in vivo via the injection and collection of liposomes [8].
| Methods of LAP enrichment | Advantages and enrichment ranges | Published applications | References |
|---|---|---|---|
| Collagenase treatment | Increased detection of LAPs by 1.3-2.2 fold. | Characterization of LAPs in adipose tissue, bone and tendon. | [9] |
| Heparin chromatography | Heparin can distinguish and enrich LAPs with small differences in glycosylation characteristics. Enrichment with heparin improved the detection of signaling proteins by 23%. | Enrichment of secreted rat brain LAPs. Fractionation of fibroblast growth factors from mouse endotheliocytes. | [10, 11]. |
| Immunoaffinity partitioning | The method applies avian polyclonal IgY antibodies or others to perform effective partitioning of protein fractions. increase in the sensitivity of 1–3 orders of magnitude | Seppro(®) IgY14 spin columns were used to deplete abundant proteins in primate serum. | [12] |
| Isopropanol extraction with polyacrylamide gel electrophoresis | Significant enrichment of LAPs. | Enhanced detection of LAPs in soybean seeds. | [13] |
| Magnetic nanoparticles for LAP enrichment | Higher protein enrichment and a low cost in comparison to ultrafiltration methods. This method provided a 1000-fold sensitivity enhancement. | Protein analysis of urine samples, detection of C-reactive protein. | [2, 14] |
| Polyethylene glycol separation and immunoaffinity-based depletion | Enhancement of LAP characterization by 43%. | Identification of LAPs in human serum. | [15] |
| Protamine sulfate precipitation | Simple, reproducible, low-cost method of depleting abundant proteins. The 0.05% protamine sulfate (PS) was sufficient to deplete major SSPs from all legumes The 0.05% protamine sulfate was sufficient to deplete the major high abundant proteins. | Depletion of abundant proteins from total seed proteins in order to enhance LAP concentration. | [16] |
| Proteomic reactor combined with pH fractionation | Enhanced LAP characterization with approximately 50%. | Characterization of LAPs in yeast extracts. | [17] |
| ProteoMiner | The method applies a large library of peptide ligands to dilute abundant proteins and enrich LAPs. The number of protein detections is significantly increased by ProteoMiner; for example, by 33% according to [18]. | Elimination of high abundance proteins in human serum, synovial fluid and urine and Pseudomonas membrane protein extracts. | [18-20] |
| Titanium dioxide (TiO2) chromatography | Titanium dioxide can enrich phosphorylated peptides and sialylated glycopeptides. | Enrichment of phosphorylated peptides and sialylated glycopeptides for mass spec analysis | [21, 22] |
A new isolation method based on collagenase treatment has been developed in order to improve MS analysis of LAPs [9]. The number of detected LAPs was increased by 1.3-2.2 fold, depending on the tissue. This technique has been successfully used for characterization of LAPs in adipose tissue, bone and tendon. In addition, the selected reaction monitoring assay has been used to improve the detection of the LAPs of bacterial origin by MS [23].
Also, another approach for the characterization of LAPs based on the proteomic reactor combined with pH fractionation has previously been described [17]. The proteomic reactor has been generated using a strong anion exchange. The obtained pH fractions have shown enhanced protein characterization with approximately 50% of fractionated proteins being LAPs as detected by mass spectrometry.
Enhanced resolution of LAPs can also be achieved by isopropanol extraction [13]. This method has been used to detect LAPs in soybean seeds. The isopropanol-based extraction has been followed by polyacrylamide gel electrophoresis (PAGE), which indicated that isopropanol at concentrations higher than 30% would provide significant enrichment of LAPs. Protein identification has been performed by mass spectrophotometry (MALDI-TOF-MS).
Furthermore, another fast and effective approach, which combines polyethylene glycol separation and immunoaffinity-based depletion, has recently been published [15]. Multiple LAPs with concentrations lower than 100 ng/mL have been identified in human serum using this technique, suggesting significant enhancement of LAP characterization. This method has raised the detection of LAPs by 43%.
Depletion of highly abundant proteins is a logical and effective approach to promote characterization of LAPs. Kim et al have applied protamine sulfate precipitation to remove abundant proteins from total seed proteins [16]. The analysis with SDS-PAGE or Western blotting of 500 μg of protein samples has shown enhanced spots in protamine sulfate-treated fraction compared to the untreated total fraction. The 0.05% of protamine sulfate was enough to remove major abundant proteins.
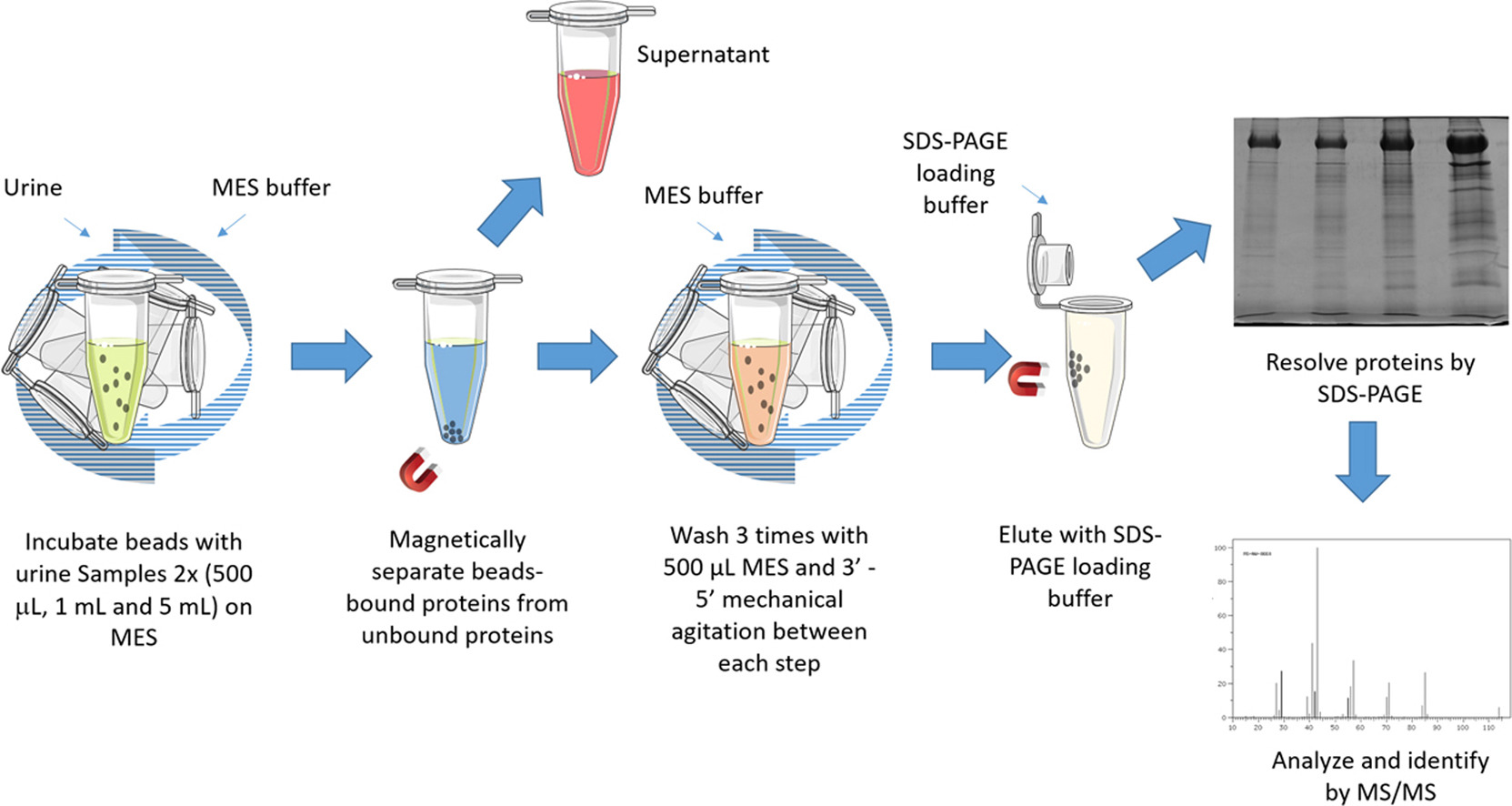
An additional platform for characterization of LAPs in biological fluids using magnetic nanoparticles (MNP) has recently been developed (Fig. 1) [2]. The method based on EDTA-functionalized nanoparticles has been applied to protein analysis of significant urine volumes and showed higher protein enrichment and a moderate cost in comparison to ultrafiltration techniques.
New methods have recently been developed to remove high abundant proteins (HAP) and enrich LAPs in biological liquids. Both MNPs and treatment with dithiothreitol and acetonitrile allowed efficient depletion of several HAPs, such as albumin and others, and amplification of the LAPs [24].
In addition, monodisperse MNPs, which were generated by gold and surfactant covering to enhance hydrophilic characteristics, have been suggested to be a very effective technique for the enrichment of LAPs [14]. The authors have tested this approach for the analysis of C-reactive protein. Notably, the sensitivity limit of monodisperse MNPs was about 1 ng per mL of the testing volume, while standard clinical cut-off concentration is usually only 1 μg per mL.
A large group of enrichment methods applies various capturing reagents, such as antibodies, specific ligands, combinations of antibodies and ligands and peptide ligand libraries [25]. ProteoMiner is another equalizing method, which is used for enrichment of LAPs by applying a comprehensive bead-based spectrum of peptide ligands, which enrich LAPs and dilute high abundance proteins. Several studies have reported the effective elimination of high abundance proteins in human serum [19], synovial fluid [18] and urine [26] and Pseudomonas membrane protein extracts [20]. Abundance equalization has also been applied for analysis of the soluble fraction of skeletal muscle [27]. In addition, immunodepletion with heavy chain IgG antibodies combined with lectin affinity chromatography has been used to enrich glycoproteins in human serum [28].
Immunoaffinity-based partitioning of high abundant proteins allows the characterization of LAPs [29]. The method applies avian polyclonal IgY antibodies to perform effective partitioning of protein fractions. Sigma provides Seppro IgY14 column system (SEP010-1KT), which is designed to eliminate the main 14 high abundant proteins. In addition, there is the SuperMix column, which can be used to separate moderately abundant proteins from LAPs.
The enrichment of LAPs may also be achieved by heparin chromatography, which is characterized by the reversible adsorption of peptides by immobilized heparins. Heparin is well known for having a strong protein binding ability and can distinguish and enrich LAPs with small dissimilarities in glycosylation characteristics. In particular, subcellular fractionation followed by heparin chromatography has been used to enrich secreted rat brain LAPs [10]. In addition, fibroblast growth factors isolated from mouse endotheliocytes have been fractionated using heparin chromatography [11]. Furthermore, heparin chromatography has been combined with protein G sepharose in order to remove highly abundant serum proteins [30].
Mass spectrometry, Western blotting, ELISA and nuclear magnetic resonance have been used to detect LAPs (Table 2).
| Methods of LAP detection | Advantages and detection range | Published applications | References |
|---|---|---|---|
| Mass spectrometry | Effective method of detection, which is often combined with various protein isolation methods. According to the recent publication, the detection limit was of 0.2 zM or approximately 120 molecules at the concentration 100 aM. | Detection of epithelial cell adhesion molecule; characterization of LAPs in adipose tissue, bone, tendon and human serum. | [1, 9] |
| Western blotting | Optimized Western blotting is effectively used for the detection of proteins with low expression levels. As an example, the new reagent AdvanBlock™-chemi allows steady detection of LAPs using 0.03-0.5 μg (Jurkat cells) or 0.2-5 μg (HeLa cells) of the whole cell lysates. | Characterization of LAPs in protein extracts from Jurkat and HeLa cells. | [31, 32] |
| ELISA | The specificity of the method is enhanced by synchronous detection of target molecules via numerous probes. Previous studies have shown that LAPs can be detected in the pg/mL ranges by ELISA. | The core Alzheimer's disease biomarkers, neurodegeneration markers and inflammation/immune modulation markers have been analyzed using ELISA. | [33, 34] |
| Nuclear magnetic resonance | This method provides high selectivity and sensitivity without a separate isolation procedure and allows the analysis of about only 6 μg of oligomers. | Analysis of amyloid-β protein. | [35] |
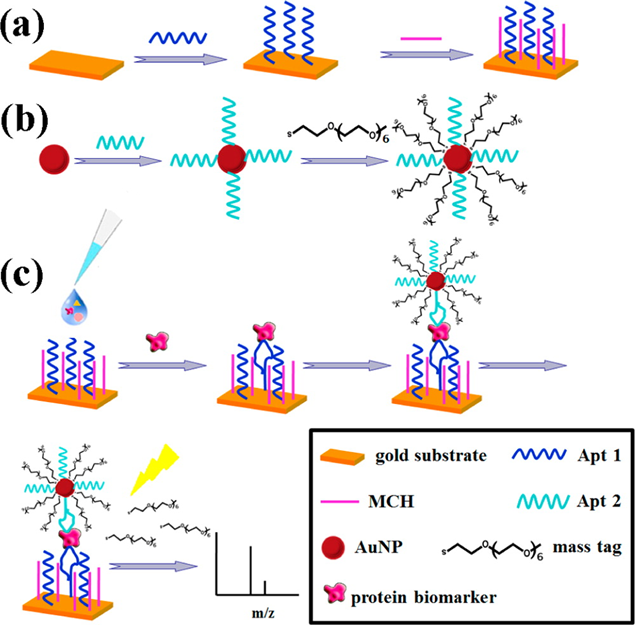
Mass spectrometry with signal augmentation is used to detect LAPs. Single-cell proteomics with mass spectrometry is an actively studied topic [36, 37]. A recent study has described the binding of LAPs to gold nanoparticles and substrates (Fig. 2) [1]. Using the detection of epithelial cell adhesion molecule (EpCAM) as an example, the study has shown that a signal can be obtained even from a very low amount of EpCAM with the detection limit of 0.2 zM or approximately 120 molecules at the concentration 100 aM.
Moreover, positive selection of target biomarkers has been shown to improve the analysis. Affinity capture reagents can improve the yield of LAPs with the concentration range 0.1-10 pg/mL [38]. The spectrum of proteins has been analyzed at a concentrations of 35 mM. The results of this method are highly tolerant of the diversity of structure and size. The authors have shown that the target molecule can be analyzed even if the oligomer constitutes just 5% of the total mixture. Moreover, this technique does not require isotopes.
Furthermore, a new method, which combined chromatography and mass spectrometry has recently been developed [39]. This method has applied iTRAQ four-plex labeling, which has allowed identification of 5300 proteins. In comparison to a label-free method, the iTRAQ four-plex labeling-based technique characterized fivefold more proteins and showed up to a nine-fold enhancement of detection throughput.
Western blotting can be used to characterize small size proteins, 5–10 μg total mass (1–3 μg total protein) [40]. The combination of Western blot with immunoprecipitation has been shown to significantly improve the identification and analysis of LAPs [31]. Furthermore, the characterization of LAPs by WB can be significantly improved by several optimization techniques. The approaches for LAP recovery include selection of appropriate lysis buffer, protein precipitation, optimization of gel electrophoresis, concentration of primary antibody and signal detection [41]. In addition, AdvanBlock™-chemi, a new blocking reagent for chemiluminescent Western blotting, has recently been used to enhance positive signal and reduce nonspecific binding [32]. This reagent allows steady detection of LAPs using 0.03-0.5 μg (Jurkat cells) or 0.2-5 μg (HeLa cells) of the whole cell lysates. The AdvanBlock™-chemi-based method has been shown to reduce nonspecific background and increase the positive specific signal by stabilizing of the antigen-antibody binding.
Since a standard ELISA is not sensitive enough to characterize LAPs, another sensitive method for LAP detection, combining ELISA with a proximity ligation assay (PLA), has been described by Tong et al [33]. Using this method, named ELISA-PLA, the specificity was enhanced by synchronous detection of target molecules via numerous probes. In addition, rolling circle amplification was applied for mending the sensitivity. Previous studies have shown that LAPs can be detected in the pg/mL ranges by ELISA [34]. Simoa technology, a variation of sandwich ELISA, can detect a target at 10-19 M level [42]. A similar assay, Proximity Extension Assay, can alos detect low levels of proteins among the bodily fluids [43]. Arboleda-Velasquez JF et al measured plasma NfL levels with an in-house simoa assay [44], so did YT Quiroz et al [45]. Holth JK et al used Simoa Human Neurology 4-Plex “A” assay and Alpha-Synuclein Discovery Kit (both from Quanterix) to measure human cerebrospinal fluid NfL, GFAP, and synuclein levels to investigate the effect of sleep deprivation on tau and amyloid beta accumulation [46]. Meso Scale Diagnostics technology can also achieve similar sensitivity. Poorbaugh J et al compared MSD S-PLEX and Quanterix SiMoA methodologies for the detection of IL-21 in human serum and plasma, and found the Limit of Detection [LOD] for both assays was approximately 1.0 fg/mL [47].
Another effective technique to characterize LAPs is nuclear magnetic resonance (NMR) with high resolution. In particular, NMR has been applied for analysis of amyloid-β protein, which is a specific marker of Alzheimer’s disease [35]. Magic angle spinning recoupling 1H-1H NMR method has allowed sub-molecular evaluation of low abundance amyloid Aβ1-40 peptide. This method in its solution- and solid-state variations allowed structural characterization of the intermediate oligomers (5-10 nm) and may be used for analysis of about only 6 μg of oligomers.
The subcellular localization of LAP can be facilitated through the "spaghetti monster" tagging of the LAP [48].
Proteins with low abundance are usually difficult to analyze without the optimization of standard methods, such as mass spectrometry or Western blotting. Therefore, most of these methods require additional signal amplification steps. Depletion of highly abundant proteins by various methods, such as collagenase, isopropanol, polyethylene glycol, protamine sulfate or nanoparticles, can be performed to enhance the specific signals for LAPs. With regard to current methods of LAP detection, which include mass spectrometry, Western blotting, ELISA and NMR, a combination with various enrichment approaches significantly improves the characterization of LAPs.
- Issaq H, Xiao Z, Veenstra T. Serum and plasma proteomics. Chem Rev. 2007;107:3601-20 pubmed
- Anderson N, Anderson N. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845-67 pubmed
- Gabriele C, Cantiello F, Nicastri A, Crocerossa F, Russo G, Cicione A, et al. High-throughput detection of low abundance sialylated glycoproteins in human serum by TiO2 enrichment and targeted LC-MS/MS analysis: application to a prostate cancer sample set. Anal Bioanal Chem. 2019;411:755-763 pubmed publisher
- de Jesus J, da Silva Fernandes R, de Souza Pessôa G, Raimundo I, Arruda M. Depleting high-abundant and enriching low-abundant proteins in human serum: An evaluation of sample preparation methods using magnetic nanoparticle, chemical depletion and immunoaffinity techniques. Talanta. 2017;170:199-209 pubmed publisher
- Castagna A, Cecconi D, Sennels L, Rappsilber J, Guerrier L, Fortis F, et al. Exploring the hidden human urinary proteome via ligand library beads. J Proteome Res. 2005;4:1917-30 pubmed
- Human IgY14 and SuperMix Columns. Available from: www.sigmaaldrich.com/technical-documents/articles/biology/seppro-igy14-system.html
- Lei T, He Q, Wang Y, Si L, Chiu J. Heparin chromatography to deplete high-abundance proteins for serum proteomics. Clin Chim Acta. 2008;388:173-8 pubmed
- Western Blot Optimization. Enhance detection & quantification of low abundance protein. Available from: www.bio-rad-antibodies.com/detect-and-quantify-low-abundant-proteins-by-western-blot.html
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Antibody Applications
- Antibody Quality
- Antibody Validation
- Loading Controls for Western Blots
- Monoclonal Antibodies - Quality Control and Quantification through Mass Spectrometry
- Quantitative Bioanalysis of Proteins by Mass Spectrometry
- Western Blot - Protocol, Troubleshooting, and Survey Results on Instruments and Reagents
reagent
