A comprehensive review of immunohistochemical markers for glia in both central and peripheral nervous systems.
Non-neuronal cells located in the central or peripheral nervous system are called glia. Glial cells provide structural and metabolic support to neurons. They can broadly be classified into macroglia and microglia. Macroglia are present under physiological conditions during both development and adulthood. There are five major classes of macroglia: astroglia, myelinating glia which include oligodendrocytes (OLs) in the CNS and Schwann cells in the PNS, NG2-glia, ependymal cells and radial glia. Astroglial cells, often referred to as astrocytes, support and nourish the cells of the brain. They maintain extracellular ion balance and assist in biochemical support of endothelial cells, maintaining the blood-brain barrier. Interestingly, astrocytes have been shown to transdifferentiate into neurons with the depletion or inhibition of the RNA-binding protein PTB [3]. One type of astrocytes is the Bergmann glia, which interacts with cerebellar Purkinje cells and aids in ion homeostasis [4]. Fañanas cells, a subset of Bergmann glia in the cerebellum, are shorter astrocytes with a feathered appearance [5]. Other astroglia include the pituicyte, which surround and regulate axon release of signaling hormones [6]. Pituicytes and Tanycytes are specialized astroglia involved in release of hormones in the hypothalamus [7]. Myelinating glia such as oligodendrocytes are responsible for the production and long-term maintenance of myelin in the central nervous system, which insulates neuronal axons and facilitates propagation of action potentials. In the peripheral nervous system, Schwann cells perform the same function, with a specialized type (nociceptive Schwann cells) initiating pain sensation [8]. NG2-glia are oligodendrocyte progenitor cells that can differentiate into other cell types depending on the environment [9]. Ependymal cells form the epithelial lining of the ventricles, the cavities of the brain containing cerebrospinal fluid, and the central canal of the spinal cord. They also give rise to the epithelial layer surrounding the choroid plexus, which produces cerebrospinal fluid, acting as an interface between the blood and the CNS. Radial glia, which are characterized by long radial processes, aid in the migration of new neurons, play a role in patterning and region-specific differentiation of the CNS, and have been identified as precursors giving rise to both neurons and glia [10, 11]. Müller glia, a type of radial glia, is critical to retinal function by providing structure and maintaining the extracellular environment for retinal neurons [12, 13]. Specific nematode glia has been shown to induce the unfolded protein response of the endoplasmic reticulum and increase longevity via neuropeptide signaling [14].
Macroglia inclusive of astrocytic and oligodendrocytic linage originate from neuroepithelial progenitor cells (NPCs), which are in embryonic neural tube and forebrain [15]. First, NPCs become radial glia. Radial glia are progenitor cells for both neurons and other glia. After neurons are created by radial glial cells, a “gliogenic switch’ leads to the radial cells becoming precursor cells for astrocytes and oligodendrocytes [16]. The subventricular zone of the hippocampus also gives rise to glial progenitor cells. Oligodendrocyte precursor cells (OPC’s) are often referred to as NG2-glia [17]. NG2-glia in the adult brain generate oligodendrocytes to maintain myelin homeostasis.
| Type | System | Location | Markers |
|---|---|---|---|
| Neural Stem cells (NSCs) | CNS | Lateral ventricle, hippocampal dentate gyrus [18] | GFAP [19] |
| Astrocyte or astroglia | CNS | Dorsal root ganglia (DRG) | GFAP [3, 20, 21] ; AQP4 [22] ; HepaCAM [23] ; ALDH1L1 [3] |
| Bergmann glial cells (type of astroglia) | CNS | Cerebellum | P75NTR [24] |
| Fañanas cells | CNS | Cerebellum | Kv2.2, KChIP3 [5] |
| Ependymal cells | CNS | Ventricles, spinal cord | FoxJ1 [25] |
| NG2-glia | CNS | Oligodendrocyte precursor cells (OPCs) | NG-2 / NG2 [3, 26] |
| Oligodendrocyte | CNS | Subventricular zone, spinal cord | OLIG2 [3] ; SOX10 [27] |
| Schwann cells | PNS | Peripheral nerve axons | SOX10, S100 β [8] |
| Satellite cells | PNS | Sensory, sympathetic, parasympathetic ganglia | GS [28] ; GFAP [21, 28] ; S100β [29] |
| Radial glia | CNS | Subgranular zone of the dentate gyrus | HOPX [30] |
| Enteric glia | PNS | Gut | GFAP [21] ; vimentin [31] |
| Pituicytes | CNS | Posterior pituitary | GFAP, S100β [32] |
| Tanycytes | CNS | Ventricle, spinal cord | Rax [33] |
| Müller glia | PNS | Retina | Rax [34] |
Glial cells located in the peripheral nervous system (PNS) originate from neural crest cells (NCC). These cells generate neurons of the dorsal root ganglia (DRG) and several types of glial cells. Both Schwann cell precursors, satellite glia and enteric glia arise from neural crest cells. Satellite glia localize to the PNS ganglia, but Schwann cell precursors move into peripheral nerves [35]. Enteric glia play a role in gut motility and function [36].
The CNS includes neural stem cells, astrocytes including Bergmann glia, Fañanas cells, NG2-glia and ependymal cells. Ependymal cells line the ventricles and have cilia [25]. NG2-glia give rise to oligodendrocyte progenitors and oligodendrocytes, which also localize to the brain. Specialized astrocytes have specific locations. For instance, pituicytes are located in the posterior pituitary, which is under the hypothalamus in the brain while tanycytes are located near the median eminence of the hypothalamus. The PNS is home to Schwann cells and enteric glia. Enteric glial cells are in the ganglia of the myenteric and submucosal plexuses of the enteric nervous system as well as in smooth muscle and mucosa tissues [37].
| Gene | Name | Glial Type | Top three suppliers |
|---|---|---|---|
| ALDH1L1 | Aldehyde dehydrogenase 1 family member L1 | Astrocytes [3] | Abcam ab177463 (2), Santa Cruz Biotechnology sc-100497 (1), Invitrogen 14-9595-82 (1) |
| AQP4 | Aquaporin 4 | Astrocyte [22, 38] | Abcam ab9512 (9), Santa Cruz Biotechnology sc-32739 (1) |
| CNPase | 2', 3'-cyclic nucleotide 3'-phosphodiesterase | Oligodendrocytes, Schwann cells | Abcam ab6319 (14), BioLegend 836404 (12), Cell Signaling Technology 5664 (5) |
| NES | Nestin | Neural progenitors [3] | BD Biosciences 611658 (21), Santa Cruz Biotechnology sc-23927 (17), Novus Biologicals NB300-266 (13) |
| GFAP | Glial fibrillary acidic protein | Neural stem cells, nonmyelinating Schwann cells, astrocytes | MilliporeSigma G3893 (248), Invitrogen 13-0300 (108), Cell Signaling Technology 3670 (74) |
| GS | Glutamine synthetase | Radial glia [39], astrocytes | BD Biosciences 610518 (24), Abcam ab64613 (8), Santa Cruz Biotechnology sc-74430 (3) |
| GT / SLC1A2 | Glutamate transporters | Astrocytes | Santa Cruz Biotechnology sc-365634 (3), Abcam ab205248 (1) |
| HOPX | HOP homeobox | Radial glia | Santa Cruz Biotechnology sc-398703 (8), MilliporeSigma WH0084525M1 (1) |
| KChIP3 | Potassium channel interacting protein 3 | Tanycytes | Santa Cruz Biotechnology sc-166916 (2) |
| Mpzl1 | Myelin protein zero-like 1 | Bergmann glia | Cell Signaling Technology 8088 (1) |
| NG2 | NG2 Chondroitin sulfate proteoglycan 4 (CSPG4) | Oligodendrocyte progenitor cells (OPCs) | Invitrogen 37-2700 (6), Novus Biologicals NB100-2688 (5), Abcam ab139406 (4) |
| OLIG2 | oligodendrocyte transcription factor 2 | Oligodendrocytes [3] ; olfactory neuronal progenitors [22] | Abcam ab109186 (15), Invitrogen MA5-15810 (1), Cell Marque 387M (1) |
| P75NTR | P75 Neurotrophin receptor | Neurons and glia | Invitrogen MA5-13311 (16), Abcam ab52987 (10), BD Biosciences 557196 (10) |
| PDGFRα | Platelet-derived growth factor α | Oligodendrocyte progenitor cells (OPCs) | Cell Signaling Technology 3174 (37), BD Biosciences 556002 (5), BioLegend 323505 (3) |
| Plp1 | Proteolipid protein 1 | Oligodendrocytes, Bergmann glia [40] | Abcam ab183493 (2), Santa Cruz Biotechnology sc-58571 (1) |
| Pmp22 | Peripheral myelin protein-22 | Oligodendrocytes, Bergmann glia [40] | Novus Biologicals NB110-59086 (2), MilliporeSigma P0081 (1) |
| PROM1 / CD133 | Prominin 1 | Neural stem cells | Miltenyi Biotec 130-090-422 (29), Cell Signaling Technology 64326 (7), BioLegend 372802 (4) |
| Rax | Retina and anterior neural fold homeobox protein | Tanycytes, Müller glia | Santa Cruz Biotechnology sc-271889 (2) |
| S100β | S100 calcium binding protein B | Glial cells, pituicytes | MilliporeSigma S2532 (64), Invitrogen MA5-12969 (57), Abcam ab52642 (50) |
| SOX2 | SRY-box transcription factor 2 | Neural progenitors | Cell Signaling Technology 3579 (67), Santa Cruz Biotechnology sc-17320 (35), Abcam ab92494 (28) |
| SOX10 | SRY-box transcription factor 10 | Glial cells, oligodendrocytes | Abcam ab155279 (23), Santa Cruz Biotechnology sc-365692 (9), Cell Marque 383R-14 (2) |
| VIM | Vimentin | Astroglia, astrocyte | Cell Signaling Technology 5741 (286), Invitrogen MA5-11883 (223), Abcam ab92547 (125) |
Without question, one of the most widely used markers of astroglial cells is GFAP (Figure 1) [41, 42]. GFAP is an intermediate filament that forms a network to provide support and strength to cells. It is thought to control their shape, movement, and function. Under conditions of injury (trauma or disease), astroglial cells rapidly increase production of GFAP [43]. More recently, several GFAP isoforms have been identified [44], including GFAPδ, which is expressed in proliferative radial glia during development as well as neural stem cells in adulthood [19]. Velasco S et al confirmed astrocytes in brain organoids with GFAP and S100B staining [45]. Z Wu et al used S100β, GFAP and glutamine synthetase as markers for astrocytes [46].
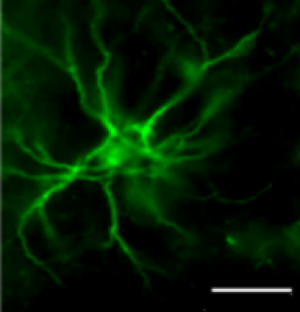
The neuroectodermal stem cell marker, or Nestin, is an intermediate filament expressed in progenitor cells. Nestin is a cytoskeletal protein that provides structural support to the cell. Nestin is found in many cell types, but often in neural stem cells or progenitor cells.
PROM1, also known as CD133, is a membrane glycoprotein expressed on adult stem cells, including neural stem cells. PROM1 suppresses differentiation and increased expression of it is associated with several types of cancer [47].
SOX10, or SRY-box transcription factor 10, is a transcription factor known to be involved in development and cellular differentiation of the neural crest cells, melanocytes, PNS neurons and glia, and CNS oligodendrocytes. It is important to neural crest cell and PNS cell differentiation as well as formation of intestinal (enteric) nerves. Sox10 induces melanocyte differentiation and is overexpressed in melanomas as well as several other cancers. Sox10 is necessary for myelination by oligodendrocytes in the CNS. Khanghahi et. al recently reported that Sox10 alone can convert astroglia into oligodendrocyte-like cells in vivo, which may lead to better treatments for diseases caused by demyelination such as multiple sclerosis [27]. Schwann cells also express Sox10 and produce myelin in the peripheral nervous system. However, Sox10 binds to different motifs in Schwann cells versus oligodendrocytes and promotes their unique differentiation [48]. Schwann cells are all recognized by the marker Sox10, but several stages of Schwann cell development have additional markers [49]. Abdo H et al detected Schwann cells with markers SOX10 and S100β [50].
Sox2 is a transcription factor expressed throughout the CNS in neural progenitor cells. Sox2 expression may be necessary to maintain the cells in a progenitor status.
Abdo H et al detected Schwann cells with markers SOX10 and S100β [50]. S100β is a protein expressed by a subtype of mature astrocytes that ensheath blood vessels and by NG2-expressing cells, which are precursors to oligodendrocytes and subpopulations of astrocytes [51, 52]. S100β has been implicated in several different functions, including neurite extension, astrocytosis, axonal proliferation, and inhibition of microtubule assembly [53, 54]. Additionally, S100β has been found to act as a neurotrophic factor during development and is elevated under circumstances of damage in adulthood [53]. Abdo H et al detected Schwann cells with S100β [50].
HOPX is a homeodomain protein but is atypical in that it lacks DNA binding sites. HOPX is expressed in diverse tissues where it regulates cell proliferation and differentiation, often contributing to tissue homeostasis. HOPX is expressed in radial astrocytes in adult dentate gyrus of the hippocampus in mice [30]. Velasco S et al stained the outer radial glia with HOPX in brain organoids [45].
Brain lipid binding protein (BLBP), also known as fatty acid binding protein 7 (FABP7), is part of the family of fatty acid binding proteins (FABPs). These proteins are localized to the nucleus and cytosol and assist with fatty acid update and metabolism [43]. BLBP is expressed radial glia in the embryonic ventricular zone and postnatal cerebellum [43]. It is found in astrocytes the postnatal subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) [55]. BLBP can be increased after an injury as astrocytes proliferate. BLBP, brain lipid-binding protein, is used as a marker for radial glia [43, 56].
The intermediate filament vimentin is a cytoskeletal component of astroglial cells. It is also expressed by radial glia and plays an important role during development. More specifically, it is thought to organize critical proteins involved in attachment, migration, and cell signaling through a phosphorylation mechanism [57]. Under conditions of injury, vimentin participates in microglia activation [58] and is associated with the migration of reactive astrocytes proximal to the site of focal injury [59]. In general, vimentin has been used reliably as a glial marker or a marker for proliferative non-neuronal cells in neuronal cell culture [60]. However, it should be noted that it has also been reported to be expressed in neurons during developmental periods and under conditions of damage [61]. Vimentin has also been reported in adult rat astrocyte cell cultures [2].
Aquaporin 4, or AQP4/AQP-4, is a water channel transmembrane protein that regulates water transport through the blood brain barrier and is expressed in the brain and spinal cord [62]. AQP4 was recently demonstrated to be elevated in a subset of embryonal tumors with multilayered rosettes (ETMRs), which are aggressive pediatric brain tumors [63]. AQP4 is found in astrocytes and ependymal cells and may be upregulated after traumatic injury [64] and translocated to cell surface in a CaM- and PKA-dependent mechanism due to hypoxia [38].
CNPase is an enzyme present in high levels in the central and peripheral nervous system. It is found almost exclusively in oligodendrocytes (and Schwann cells) [65] and is thought to be important in CNS myelination [66]. CNPase appears early during development in oligodendrocytes, compared with many other myelin proteins and continues to be expressed in oligodendrocytes of adult animals [67]. The membrane-bound protein can link tubulin to membranes and may also regulate cytoplasmic microtubule distribution [68].
The p75 neurotrophin receptor is a transmembrane receptor present in both glial and neuronal cells. P75NTR has two major functions. By itself, p75NTR can activate signaling that induces apoptosis or instead promotes cell survival. By interacting with Trk family tyrosine kinases, p75NTR may act as a co-receptor and enhance or diminish neurotrophin-mediated Trk receptor activity. The functions of p75NTR in glia was reviewed by Cragnolini and Friedman in 2008 [69]. Recently, Koike et. al identified a new type of glial cell located in the DRG that is positive for p75 neurotrophin receptor [24].

Glutamate transporter proteins bind L-glutamate, which is a major neurotransmitter in the CNS. Uptake of glutamate from the synapse by glutamate transporters within astrocytes maintain the concentration of synaptic glutamate. The glutamate transporters are critical to maintaining the glutamate concentrations to normal physiological status because when disrupted, it leads to injury and degeneration. EAAT1 is the major glutamate transporter in cerebellar astrocytes [70]. GLT-1 is present in Bergmann glia processes
Glutamate is taken up by astrocytes via the glutamate transporter, and then is converted into glutamine by the glutamate synthetase (GS) [71]. GS catalyzes a reaction including ammonia and glutamate and required ATP. GS is critical to glutamate detoxification. Glutamate synthetase is enriched in astrocytes [46], as demonstrated by Figure 2 [2].
NG2 chondroitin sulfate proteoglycan 4 (CSPG4) is a marker of NG2 glia [46], which are also knowns as oligodendrocyte progenitor cells or OPCs. NG2-CSPG4 is a very large transmembrane protein known to induce signaling cascades both internally and outside the cell. CSPG4 has been shown to inhibit neurite and axon growth [72]. Knockout of CSPG4 in brain injury studies have demonstrated that it is necessary to improve inflammation and tissue loss after an injury [73].
Retina and anterior neural fold homeobox (Rax) protein is a transcription factor expressed in tanycytes of the hypothalamus and within Müller glia of the eye [33, 74]. Rax knockout mice have no eye formation [75].
Potassium voltage-gated channel subfamily B member 2 (Kv2.2) is a protein ion channel that regulates neurotransmitter release. Kv2.2 is expressed on many cells, including the Fañanas cells of the cerebellum [5].
Potassium channel interacting protein 3 (KChIP3), also known as Downstream regulatory element antagonistic modulator (DREAM) or calsenilin (CSEN), regulates potassium channel activity by binding calcium, DNA, and other proteins. KChIP3 was recently shown to identify Fañanas glia in the cerebellum [5].
IC Clark et al stained astrocytes with EphB3 as a marker in flow cytometry [76]. E Gelpi et al proposed to use nuclear p62 staining to identify Alzheimer type II astrocytes in metabolic/hepatic encephalopathy [77].
Dr. Patima Tanapat wrote the first version of this article as part of the article Neuronal Cell Markers in 2013. Dr. Jennifer Walker-Daniels made a major revision in Dec 2019.
- Hatton G. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67-79 pubmed
- Wittkowski W. Tanycytes and pituicytes: morphological and functional aspects of neuroglial interaction. Microsc Res Tech. 1998;41:29-42 pubmed
- Singec I, Knoth R, Ditter M, Hagemeyer C, Rosenbrock H, Frotscher M, et al. Synaptic vesicle protein synaptoporin is differently expressed by subpopulations of mouse hippocampal neurons. J Comp Neurol. 2002;452:139-53 pubmed
- Franze K, Grosche J, Skatchkov S, Schinkinger S, Foja C, Schild D, et al. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A. 2007;104:8287-92 pubmed
- Ma D, Ming G, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514-20 pubmed
- Eng L, Ghirnikar R, Lee Y. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res. 2000;25:1439-51 pubmed
- Albuerne M, Mammola C, Naves F, Levanti B, Germana G, Vega J. Immunohistochemical localization of S100 proteins in dorsal root, sympathetic and enteric ganglia of several mammalian species, including man. J Peripher Nerv Syst. 1998;3:243-53 pubmed
- Jessen K, Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983;3:2206-18 pubmed
- Furukawa T, Mukherjee S, Bao Z, Morrow E, Cepko C. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383-94 pubmed
- Feng L, Hatten M, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895-908 pubmed
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707-16 pubmed
- Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol. 2007;81:108-18 pubmed
- Deinum J, Baudier J, Briving C, Rosengren L, Wallin M, Gerard D, et al. The effect of S-100a and S-100b proteins and Zn2+ on the assembly of brain microtubule proteins in vitro. FEBS Lett. 1983;163:287-91 pubmed
- Ivaska J, Pallari H, Nevo J, Eriksson J. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050-62 pubmed
- Badaut J, Lasbennes F, Magistretti P, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367-78 pubmed
- Wang K, Bekar L, Furber K, Walz W. Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res. 2004;1024:193-202 pubmed
- Sprinkle T. 2',3'-cyclic nucleotide 3'-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235-301 pubmed
- Gravel M, Peterson J, Yong V, Kottis V, Trapp B, Braun P. Overexpression of 2',3'-cyclic nucleotide 3'-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol Cell Neurosci. 1996;7:453-66 pubmed
- Takatsuru Y, Iino M, Tanaka K, Ozawa S. Contribution of glutamate transporter GLT-1 to removal of synaptically released glutamate at climbing fiber-Purkinje cell synapses. Neurosci Lett. 2007;420:85-9 pubmed
- Fidler P, Schuette K, Asher R, Dobbertin A, Thornton S, Calle Patino Y, et al. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci. 1999;19:8778-88 pubmed
- Furukawa T, Morrow E, Cepko C. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531-41 pubmed
- Mathers P, Grinberg A, Mahon K, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603-7 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- gene
- human 2 3 cyclic nucleotide 3 phosphodiesterase
- human ACADSB
- human CD133
- human FABP7
- human GFAP
- human HEPACAM
- human HOPX
- human KCNB2
- human KCNIP3
- human MAP2
- human NEUROD1
- human NG2
- human NSE
- human NeuN
- human OLIG2
- human PDGFR alpha
- human PLP1
- human PMP22
- human PZR
- human RAX
- human S100B
- human SLC1A2
- human SOX10
- human SOX2
- human SYNPR
- human TUJ1
- human aquaporin 4
- human c-Fos
- human calbindin
- human calretinin
- human doublecortin
- human formyltetrahydrofolate dehydrogenase
- human glutamine synthetase
- human nestin
- human neurofilament L
- human p75NTR
- human tau
- human vimentin
