Here we present a comprehensive review of laboratory detergents and their applications in biomedical experiments. This review includes discussions of ionic, non-ionic and zwitterionic detergents, their general properties as well as information about commonly used detergents from each group. Finally, we include a brief discussion of Labome survey results for some common detergents.
Detergents used in biomedical laboratories are mild surfactants (surface acting agents), used for cell lysis (i.e., the disruption of cell membranes) and the release of intracellular materials. They are amphiphilic molecules, containing both hydrophilic and hydrophobic regions. This amphiphilic property allows detergents to break protein-protein, protein-lipid and lipid-lipid associations, denature proteins and other macromolecules, and prevent nonspecific binding in immunochemical assays and protein crystallization.
There are many types of detergents used in laboratory research. New amphiphilic compounds, usually designed for specific applications, continue to be developed (e.g., maltose-neopentyl glycol [1] and glycosyl-substituted dicarboxylates [2] ). This article reviews the characteristics and applications of the most commonly used laboratory detergents.
| Type | Chemicals |
|---|---|
| ionic | sodium dodecyl sulfate (SDS), deoxycholate, cholate, sarkosyl |
| non-ionic | Triton X-100, DDM, digitonin, tween 20, tween 80 |
| zwitterionic | CHAPS |
| chaotropic | urea |
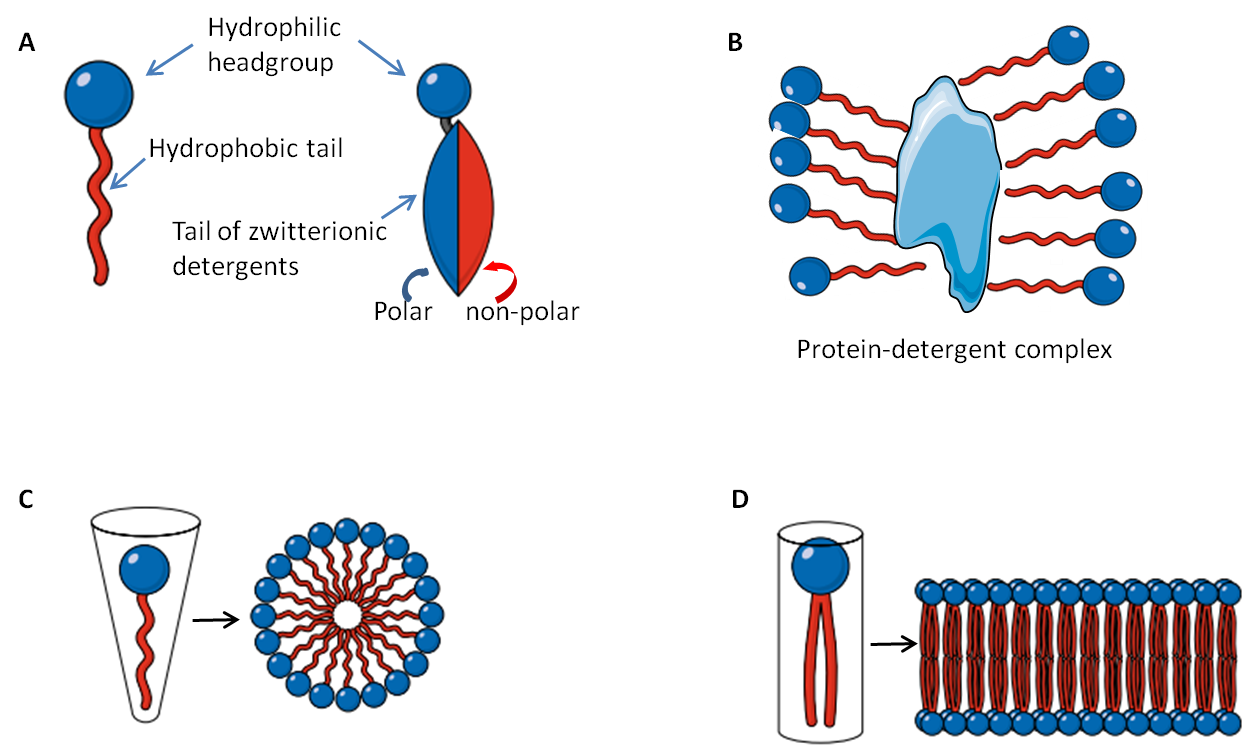
Detergents are amphiphilic organic compounds comprised of a hydrophobic non-polar hydrocarbon moiety (tail) and a hydrophilic polar headgroup (Fig. 1A). This molecular structure is very similar to the amphiphilic phospholipids that make up our cellular membranes, except that the phospholipids possess pair hydrophobic tails attached to the hydrophilic headgroup (Fig 1D). When dissolved in water at appropriate concentrations and temperatures amphiphilic molecules self-assemble into structures that keep their hydrophilic headgroups on the exterior and the hydrophobic tails on the interior away from the water. Due to their molecular differences, detergent molecules form spherical micelles(Fig. 1C) while phospholipids are more likely to develop a bilayer (Fig 1D). The similarity in molecular structures allows the detergent to penetrate phospholipid bilayers and thus disrupt cell membranes.
Furthermore, the hydrophobic core of the micelle can bind to hydrophobic regions of proteins (Fig 1B). The number of detergent molecules in a micelle is called the aggregation number, an important parameter used to assess membrane protein solubility [3]. The length of the hydrophobic region is directly proportional to the degree of hydrophobicity, and it is quite constant among detergents, while the charged headgroup is variable. Both temperature and concentration are important parameters of phase separation and solubility of a detergent. The minimal detergent concentration at which micelles are observed at a given temperature is called the Critical Micelle Concentration (CMC). At any concentrations lower than the CMC, only monomers are observed; at concentrations higher than CMC both micelles and monomers co-exist, along with other non-micellar phases that are not dissolved in water. Likewise, the lowest temperature at which micelles are formed is called Critical Micelle Temperature (CMT). CMC is also affected by the degree of lipophilicity of the headgroup. Generally, a low lipophilic or lipophobic character results in high CMC.
Common detergents are categorized into three groups based on their characteristics: ionic (anionic or cationic), non-ionic and zwitterionic. Below I discuss common detergents in each of these categories and provide important information about the selection and use of laboratory detergents.
Ionic detergents are comprised of a hydrophobic chain and a charged headgroup which can be either anionic or cationic. They generally have higher CMC values than non-ionic detergents and tend to be fairly harsh. Due to their charged headgroups, ionic detergents cannot be removed by ion exchange chromatography. Furthermore, additional precautions should be taken when using ionic detergents because some of their properties may be altered in buffers with variable ionic strength (e.g., CMC can fall dramatically when the NaCl concentration increases from 0 to 500 mM).
The anionic SDS is a very commonly used and effective surfactant in solubilizing most proteins. It disrupts non-covalent bonds within and between proteins, denaturing them, and resulting in the loss of their native conformation and function. SDS binds to a protein with a ratio of 1.4:1 w/w (corresponding to about one SDS molecule per two amino acids), masking the charge of the protein. Thus SDS adds an overall negative charge to all proteins in the sample regardless of their isoelectric point (pI). Once bound by negatively charged SDS molecules the proteins can be separated based on size. That is a big reason for the wide use of SDS polyacrylamide gel electrophoresis (SDS-PAGE) for separating and studying proteins. Usually, for complete cell lysis in the presence of SDS, a sample must be sonicated or sheared (e.g., passed through a 19G needle) several times to ensure DNA degradation. SDS cannot be used when active proteins are required or when protein-protein interactions are being studied because both of these are disrupted by the SDS. When working with SDS it is important to know that SDS precipitates at low temperatures, and this effect is enhanced in the presence of potassium salts. This phenomenon can sometimes be exploited to remove SDS from a protein sample [4]. SDS can be used for decellularization [5].
Sodium deoxycholate and sodium cholate are bile salts detergents. They are both anionic detergents. These detergents are often used for membrane disruption and membrane protein extraction, for example, apelin receptor [6]. Deoxycholate does denature proteins while cholate is a non-denaturing detergent. One potential benefit to both of these detergents is that they can be removed from samples via dialysis, which may help with quantification and/or downstream analyses of proteins.
Sarkosyl, also known as sarcosyl or sodium lauroyl sarcosinate, is an anionic surfactant. It is amphiphilic due to the hydrophobic 14-carbon chain (lauroyl) and the hydrophilic carboxylate. The carboxylate with a pKa value of 3.6 is negatively charged in any physiological solution. Sarkosyl is prepared from lauroyl chloride and sarcosine in the presence of sodium hydroxide and is purified by recrystallization from alcohol, or by acidification with a mineral acid, separation of the free acid, and neutralization of the free acid. Sarkosyl has also been used to improve wetting and penetration of topical pharmaceutical products. In the food industry, sarkosyl is approved for use in processing, packaging, and transporting food for human consumption, and in adhesives used in food storage or transportation. It is widely used in cosmetic formulations such as shampoos and body washes at concentrations around 3-13% [7]. Sarkosyl is also used in metal finishing end processing for its crystal modifying, anti-rust, and anti-corrosion properties.
Sarkosyl is widely utilized in laboratory experiments, for example for solubilizing tau in Alzheimer disease research [8], due to its good water solubility, high foam stability, and strong sorption capacity to proteins. Sarkosyl serves as a detergent to permeabilize cells and extract proteins in isolation and purification techniques such as western blot and indirect ELISA. It can also inhibit the initiation of DNA transcription.
One major application of sarkosyl is for solubilizing and refolding proteins from inclusion bodies (protein aggregates within cytoplasm or nuclei). Eukaryotic recombinant proteins overexpressed in Escherichia coli tend to form such inclusion bodies. Sarkosyl is often used to solubilize an inclusion body pellet to extract the proteins and allow them to refold into their native form. Earlier work involved solubilizing inclusion bodies with denaturants, such as urea or guanidinium hydrochloride, and refolding by slow dilution — however, most of the solubilized proteins aggregate and precipitate upon removal of the strong detergents. Sarkosyl is an effective solubilizing agent that minimizes aggregation and allows refolding at higher protein concentrations (as much as 10-fold higher when compared to using guanidinium hydrochloride [9] ). One study found the over 95% of inclusion body fusion proteins were solubilized with 10% sarkosyl, and that the proteins could then be recovered with a mix of other detergents (i.e., Triton X-100 and CHAPS) [10]. Proteins in the soluble extract with sarkosyl can also be stored at 4°C for a week before affinity purification. It should be noted, however, that sarkosyl interferes with the subsequent chromatographic process and must be removed from the solution by dilution or dialysis.
Non-ionic detergents have uncharged hydrophilic headgroups. They are considered mild surfactants as they break protein-lipid and lipid-lipid associations, but typically not protein-protein interactions, and generally, do not denature proteins. Therefore, many membrane proteins may be solubilized in their native and active form, retaining their protein interactors. However, because not all proteins behave the same with different non-ionic detergents, trial and error may be necessary to find the best detergent for your protein(s) of interest. Additionally, it should be noted that most non-ionic detergents interfere with ultra-violet (UV) spectrophotometry. Therefore, protein determination at 280 nm in the presence of non-ionic detergents is typically imprecise.
All members of the Triton family: Triton X-100, Triton X-114, Nonidet P-40 (NP-40), Igepal® CA-630, are quite similar, differing slightly in their average number (n) of monomers per micelle (9.6, 8.0, 9.0, and 9.5, respectively) and the size distribution of their polyethylene glycol (PEG)-based headgroup. The CMC values of these detergents are low, and therefore they can not be easily removed by dialysis. Triton X-100, a typical non-ionic detergent, derives from polyoxyethylene and contains an alkylphenyl hydrophobic group. Triton X-100 is commonly used for isolating membrane protein complexes, and the surfactant of choice for most such as for co-immunoprecipitation experiments. Other members of the Triton family are used for membrane protein isolation by phase-separation due to low cloud points (the temperature at which the micelles aggregate and form a distinct phase). While the cloud point of Triton X-100 is 64°C, the cloud point of Triton X-114 is 23°C. This allows for membrane protein extraction and solubilization in Triton X-114 without bringing the samples up to warmer temperatures which may denature many proteins.
Brij™ 35 is another nonionic polyoxyethylene surfactant, commonly used as a component of cell lysis buffers or assay buffers or a surfactant in HPLC applications.
The n-dodecyl-β-D-maltoside (DDM) is a glycosidic surfactant, increasingly used with hydrophobic and membrane protein isolation when the protein activity needs to be preserved. It is more efficient at protein solubilization for 2-D electrophoresis than several other detergents, including CHAPS and NP-40 [11]. The glycochain in its lipophilic site, its high CMC of 0.17 mM and the interface of the micelles create an aqueous-like microenvironment ideal for solubilizing and retaining the stability of membrane and hydrophobic proteins [12]. For example, Winkler MBL et al purified NCR1 protein with the addition of n-dodecyl-β-D-maltopyranoside [13] ; so did Li Y et al for LptB2FG and LptB2FGC proteins [14]. Steichen JM et al mixed protein complexes in a solution of DDM from Anatrace before Cryo-EM [15]. DDM is also used as an excipient in nasal sprays Valtoco and Tosymra.
Other maltosides, such as beta-decyl-maltoside, have different lengths of the hydrophobic alkyl chains. Glucoside (octyl-glucoside) are a potential alternative to maltoside detergents for protein research [16].
Digitonin, a steroidal glycoside derived from the purple foxglove plant (Digitalis purpurea), is used for the solubilization of cellular membranes. As with other non-ionic detergents discussed here, digitonin is frequently used to solubilized membrane proteins without denaturing them. For example, B de Laval et al lysed cells with 1% of digitonin for Tn5 transposase reaction during the ATAC-seq protocol [17]. Zhao Y et al released synaptic and extrasynaptic AMPA receptors from postsynaptic density with digitonin [18]. Additionally, digitonin is used to extract cellular organelles. Digitonin interacts with cholesterol in membranes and thus can be used to permeabilize the cholesterol-rich plasma membrane while leaving the cholesterol-poor organelle membranes intact.
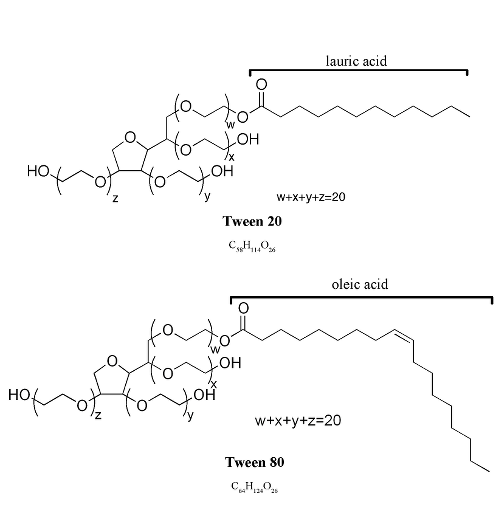
Tween-20 and Tween-80 are polysorbate surfactants with a fatty acid ester moiety and a long polyoxyethylene chain. They have very low CMC, are generally gentle surfactants, do not affect protein activity and are effective in solubilization. Tweens are not common ingredients of cell lysis buffers; however, they are routinely used as washing agents in immunoblotting and ELISA to minimize nonspecific binding of antibodies and to remove unbound moieties, and used to permeabilize cell membranes. For example, Yang J et al immunostained intracellular FLAG tag after treating HEK293 cells with 0.2% Tween 20 [19]. The solubilization of lipid membranes by Tween-20 is studied in detail recently [20].
One common question regarding the Tween family detergents is the difference between Tween 20 and Tween 80, the two most commonly used members. Tween 20 has lauric acid, while Tween 80 has oleic acid (Figure 3). Table 2 summarizes various aspects between them. These detergents can often be used interchangeably; however, the difference between them is sometimes important, such as in in vivo studies that may be influenced by the different levels of hemolytic effect of Tween 20 and Tween 80 [21]. Greenwood DJ et al, for example, grew Mycobacterium tuberculosis in a medium supplemented with 0.05% Tween 80 [22]. Ouadah Y et al injected a dibenzazepine solution with 0.1% v/v Tween 80 into mice to inhibit Notch signalling [23].
| Synonyms | Chemical Formula | Molecular Weight | Density (g/mL) | Appearance | Applications | |
|---|---|---|---|---|---|---|
| Tween 20 | polysorbate 20, polyoxyethylene sorbitan monolaurate, PEG (20) sorbitan monolaurate | C58H114O26 | 1228 | 1.1 | Clear, yellow to yellow-green viscous liquid | a broad range of applications: as a blocking agent in PBS or TBS wash buffers for ELISA, Western blotting and other immunoassay methods; for lysing mammalian cells; and as a solubilizing agent for membrane proteins. |
| Tween 80 | polysorbate 80, polyoxyethylene sorbitan monooleate, PEG (80) sorbitan monooleate | C64H124O26 | 1310 | 1.06-1.09 | amber colored viscous liquid | as a stabilizing agent for proteins; used in tests for the identification of phenotype of some mycobacteria; used in vaccine preparations [24] |
Though non-ionic detergents are generally relatively mild, many proteins do denature or aggregate in the presence of these detergents. To ameliorate this issue new non-ionic glyco-lithocholate amphiphiles (GLC-1, GLC-2, and GLC-3) and glyco-diosgenin amphiphile (GDN) have been developed [25]. GDN was used to extract yeast mitochondrial dimeric ATP synthase to understand better how the protein functions [26]. Pluronic F-68 is commonly used in suspension cell culture at 0.1% to reduce the water shear force [27].
The headgroups of zwitterionic detergents are hydrophilic and contain both positive and negative charges in equal numbers, resulting in zero net charge. They are more harsh surfactants than the non-ionic detergents. A typical zwitterionic detergent is 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, better known as CHAPS. CHAPS high CMC (6 mM at room temperature) allows efficient removal by dialysis. It is very common in sample preparation at concentrations of 2-4% for isoelectric focusing and 2D electrophoresis. CHAPSO differs with CHAPS in that it contains a more polar headgroup, which makes it more capable of solubilizing hydrophobic molecules. Thus, CHAPSO is mainly used for solubilization of integral membrane proteins.
Chaotropic agents are similar substances to surfactants in that they break non-covalent interactions (hydrogen bonds, dipole-dipole interactions, hydrophobic interactions) facilitating protein denaturation, which in this case is usually reversible. Urea is a common chaotropic agent used alone, or in combination with thiourea or other detergents, in applications like 2D-gel electrophoresis and in-solution enzymatic digestion of proteins for preparation during proteomic workflows. When using Urea, extra care must be taken not to heat the sample above 37°C as this will lead to carbamylation of proteins [28].
For membrane protein solubility, a detergent with high CMC should generally be chosen, and the volume and concentration of the buffer are also crucial as enough detergent should be present to solubilize all membrane proteins in the sample. In most cases, the detergent concentration should be well about the CMC level (at least 2X the CMC) to ensure sufficient micelle concentration to solubilize the membrane proteins. According to Linke [3], at least one micelle is needed per membrane protein molecule to sufficiently mimic the lipid environment of a membrane (Fig. 1B, D).
Phase separation can be used to purify the proteins further. This requires adjusting the temperature and the concentrations of salts and detergent in the buffer to cause the detergent micelles to aggregate and separate from the aqueous layer. In this case, the membrane proteins, surrounded by the micelles, aggregate with the detergent. The temperature at which the detergent solution separates into two phases, the cloud point, is affected by glycerol or salts in the buffer (e.g., Triton X-114 has a cloud point of 23°C, but in the presence of 20% glycerol, the cloud point declines to 4°C). This is very important since the stability of a protein is affected by high temperatures.
A good detergent should be able to lyse cells, solubilize proteins and be suitable for your downstream application(s). Also, the solubilized protein in native or denatured form should be considered. There is no ideal detergent for all applications, and even in the same application, the result varies (Table 3). Therefore, after options are considered, trial and error are often necessary to find the best detergent, and a mixture of detergents may be optimal. Also, the fresh preparation of detergent working solution is usually the best practice to avoid hydrolysis and oxidation.
| Detergent | MW (Da) monomer | MW (Da) micelle | CMC (mM) 25oC | Aggregation No. | Cloud Point (oC) | Avg. Micellar Weight | Strength | Dialyzable | Applications |
|---|---|---|---|---|---|---|---|---|---|
| SDS | 289 | 18,000 | 7-10 | 62 | >100 | 18,000 | Harsh | Yes | Cell lysis, Electrophoresis, WB, hybridization |
| Triton X-100 | 625 | 90,000 | 0.2-0.9 | 100-155 | 65 | 80,000 | Mild | No | Enzyme immunoassays, IP, Membrane solubilization |
| CHAPS | 615 | 6,150 | 6 | 10 | >100 | 6,150 | Mild | Yes | IEF, IP |
| NP-40 | 680 | 90,000 | 0.059 | 45-50 | Mild | No | IEF | ||
| n-dodecyl-β-D-maltoside | 511 | 0.15 | 98 | 50,000 | Protein Crystallization | ||||
| Tween-20 | 1228 | 0.06 | 76 | Mild | No | WB, ELISA, Enzyme immunoassays | |||
| Digitonin | 1229 | 70,000 | <0.5 | 60 | 70,000 | Mild | No | Membrane solubilization |
The downstream applications often require that detergent concentrations be lowered or completely removed. For such purposes, size exclusion chromatography or dialysis can be used if the micelle size is substantially different than the protein of interest or micelles are small enough (i.e., high CMC) to pass through the dialysis tubing [3]. Other methods employ the use of detergent binding non-polar beads or resins, cyclodextrin inclusion compounds [29], ion-exchange chromatography or protein precipitation. However, the buffer used after detergent removal must be selected carefully to avoid protein precipitation or aggregation.
Labome surveys the literature for the application of detergents. The following table lists the main suppliers, and the number of articles, indicating most of the detergents are supplied by MilliporeSigma.
| detergent | suppliers | |
|---|---|---|
| Triton X-100 | MilliporeSigma [30], Thermo Fisher [31, 32], Electron Microscopy Sciences [33] | |
| Tween-20 | Bio-Rad [34], MilliporeSigma [32], Thermo Fisher | |
| SDS | Amresco, Bio-Rad, Q.BIOgene, MilliporeSigma | |
| NP-40 | Roche, MilliporeSigma [35] | |
| CHAPS | MilliporeSigma, JT Baker | |
| digitonin | MilliporeSigma, Wako | |
| DDM | Generon [36], Anatrace [16] |
Thermo Fisher Pierce Triton X-100, for exampl, BP151 [32] or 85111 [31], was used to lyse cell and tissue samples for immunohistochesmitry [32] and immunocytochemistry [31]. MilliporeSigma Triton X-100 was used to lyze cells [37], or permeabilize cells in immunocytochemistry [38], and in blocking buffer for immunohistochemistry [39, 40] and proteinase K protection assay [41].
Tween-20 is commonly used in washing buffers, such as TBS-Tween (TBS-T) or PBS-Tween (PBT-T), in various immunoassays. MilliporeSigma Tween-20, for example, P1379 [32], was used in washing blots [32], in IHC experiments (P1379) [42], in immunoprecipitation [43],and in microfluidic array multiplex PCR [44] and others [45]. MilliporeSigma Tween-80, was used to dissolve erlotinib (a chemotherapy drug) [46] and as a supplement to grow M. tuberculosis strains [47].
Lonza SDS (catalog number 51213) was used in chromatin preparations [42]. Amresco SDS was used in SDS-PAGE [48]. Bio-Rad sodium dodecyl sulfate was used to prepare a radioimmunoprecipitation assay buffer [49]. MilliporeSigma-Aldrich SDS was used to prepare buffers for, among others, in vitro octanoylation assays, Laemmli sample buffer, 2D-DIGE experiments [50].
Roche NP-40 was used in cell lysis [51, 52]. MilliporeSigma NP-40 was used to prepare radioimmunoprecipitation assay buffer [49], cell lysis/homogenization buffers buffer [53, 54] and immunoprecipitation assay RIPA buffer [55].
MilliporeSigma CHAPS was used in buffers for protein crystallization [56]. JT Baker CHAPS was used to lyse cells to study viral interaction with human ASF1 protein [57].
MilliporeSigma was used in an immunocytochemistry experiment to study PI4P [58] and used to perform proteinase K protection assays [41], and to extract RNA [59]. Wako digitonin was used to lyse cells [60] and perform immunoprecipitation experiments [61].
Y Lee et al solubilized a GPCR protein with dodecylmaltoside / DDM from Generon [36]. Anatrace n-decyl-beta-D-maltopyranoside was used for protein purification [62, 63] ; so were its n-dodecyl-beta-D-maltoside [64] and n-undecyl-beta-D-maltoside [65, 66]. Glycon beta-dodecyl-maltoside and beta-decyl-maltoside were also used in protein purification [67]. Anatrace n-octyl-beta-glucoside was used in solubilizing AQP4 proteins [16].
Silva MC et al used Brij-35 as the detergent for bio-layer interferometry biosensor assay [68]. For protein purifications, Affymetrix octyl glucose neopentyl glycol (OGNPG) at 1% [69], and MilliporeSigma cholesteryl hemisuccinate at 0.1% or 0.05% (w/v) [12, 70] were used. For chromatin-related assays, MilliporeSigma-Aldrich sodium deoxycholate (catalog number D6750) and Igepal (catalog number I8896), and TEKnova N-lauroylsarcosine (catalog number S3379) were used [42].
- Suzuki H, Terada T. Removal of dodecyl sulfate from protein solution. Anal Biochem. 1988;172:259-63 pubmed
- Lanigan R. Final report on the safety assessment of Cocoyl Sarcosine, Lauroyl Sarcosine, Myristoyl Sarcosine, Oleoyl Sarcosine, Stearoyl Sarcosine, Sodium Cocoyl Sarcosinate, Sodium Lauroyl Sarcosinate, Sodium Myristoyl Sarcosinate, Ammonium Cocoyl Sarcosinate,. Int J Toxicol. 2001;20 Suppl 1:1-14 pubmed
- Burgess R. Purification of overproduced Escherichia coli RNA polymerase sigma factors by solubilizing inclusion bodies and refolding from Sarkosyl. Methods Enzymol. 1996;273:145-9 pubmed
- Luche S, Santoni V, Rabilloud T. Evaluation of nonionic and zwitterionic detergents as membrane protein solubilizers in two-dimensional electrophoresis. Proteomics. 2003;3:249-53 pubmed
- Vinardell M, Infante M. The relationship between the chain length of non-ionic surfactants and their hemolytic action on human erythrocytes. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:117-20 pubmed
- Degrip W, VanOostrum J, Bovee Geurts P. Selective detergent-extraction from mixed detergent/lipid/protein micelles, using cyclodextrin inclusion compounds: a novel generic approach for the preparation of proteoliposomes. Biochem J. 1998;330 ( Pt 2):667-74 pubmed
- Peralta Ramirez J, Hernandez J, Manning Cela R, Luna Munoz J, Garcia Tovar C, Nougayrede J, et al. EspF Interacts with nucleation-promoting factors to recruit junctional proteins into pedestals for pedestal maturation and disruption of paracellular permeability. Infect Immun. 2008;76:3854-68 pubmed publisher
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- reagentmethod
- Antibody Applications
- Assay Development: 5 Considerations and 8 Fundamentals
- DNA Extraction and Purification
- Flow Cytometry and Cell Sorting: A Practical Guide
- Nanodiscs: Membrane Protein Research in Near-Native Conditions
- Optical Clearing of Biological Tissue
- Organelle Markers
- Protein Purification
- Protein Quantitation
- Receptor-Ligand Binding Assays
- Subcellular Fractionation
- Tumor Markers Currently Utilized in Cancer Care