This article reviews anti-nucleotide and anti-nucleic acid antibodies commonly used in biomedical laboratory experiments.
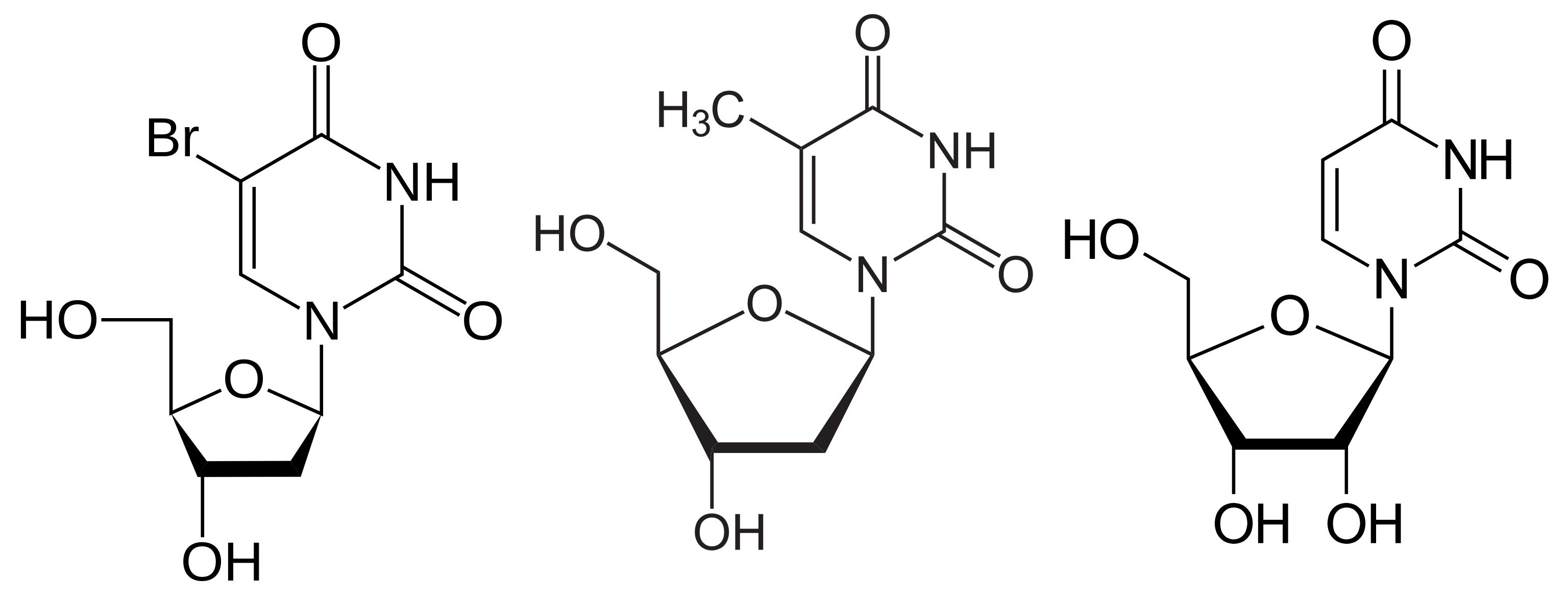
BrdU (bromodeoxyuridine, 5-bromo-2'-deoxyuridine, BUdR, BrdUrd, broxuridine), an analog of deoxythymidine (Fig 1), can be incorporated into DNA by DNA polymerase during the S phase of the cell cycle, and has been used to indicate cell proliferations. BrdU within DNA molecules is recognized by several clones of BrdU antibodies. Table 1 lists these clones, their applications and recent references among the publications Labome has surveyed. These antibodies have been used in a variety of immuno methods - immunohistochemistry, immunocytochemistry, flow cytometry, immunoprecipitation, and Western blotting. BrdU assays are routinely used to measure cell proliferation. Detection of BrdU by BrdU antibodies usually require either acid or enzymatic or copper ion treatment of chromatins to expose BrdU. Commercial kits, such as APC BrdU Flow Kit from BD, are commonly used [1].
BU1/75(ICR1) clone is a rat monoclonal antibody, of IgG2a subcless, and specific against bromouridine and chlorouridine, but not against iodouridine and thymidine [2]. It is the widely used BrdU antibody in the literature Labome has surveyed. Yu K et al injected BrdU solution intraperitoneally into mice and stained the frozen sections from the mice to assay in vivo cell proliferation [3]. Sarek G et al, for example, stained mouse ear fibroblasts pulse-labelled with CldU with this rat anti-BrdU antibody from AbD Serotec (now Bio-Rad) [4]. M Coolen et al quantitated proliferating BrdU+ cells in killifish pallium with this clone [5].
Gonchoroff NJ et al injected Balb/c mice with 5-iodouridine covalently coupled to ovalbumin, fused the spleen cells with the P3/NSl/l-Ag4-1 mouse myeloma, and selected clones reactive with bromouridine and iodouridine but nonreactive with ovalbumin [6]. The resultant clone BU-1, of IgG2a subclass, has a dissociation constant of 80 nM for free bromouridine. It is reactive against bromouridine, chlorouridine, iodouridine, but not fluorouridine and thymidine. Unlike clone B44, BU-1 antibody can detect BrdU in DNA without denaturation [6].
Gratzner HG immunized BALB/c mice with a conjugate of iodouridine and ovalbumin, fused the resultant spleen cells the plasmacytoma line SP2/0Ag14, and selected a clone (B44) highly specific for bromodeoxyuridine and iododeoxyuridine with no cross-reaction with thymidine [6]. This clone can detect BrdU in denatured DNA [6]. Sarek G et al, for example, stained mouse ear fibroblasts pulse-labelled with IdU with this mouse anti-BrdU antibody from BD [4].
G3G4 is a mouse IgG1 clone, deposited by Dr. SJ Kaufman to DSHB in 1993.
| Clone | Application | Supplier, catalog number, and reference |
|---|---|---|
| BU1/75 | ChIP | Abcam ab6326 [7] |
| FC | Abcam ab6326 [8] ; Accurate Chemical OBT0030 [9] | |
| IC | Abcam ab6326 [10] ; Accurate Chemical OBT0030 [11] | |
| IHC-F | Abcam ab6326 [3, 12] | |
| IHC-Free | Abcam ab6326 [13] ; Accurate Chemical OBT0030 [14] | |
| IHC-P | Abcam ab6326 [15] ; Accurate Chemical OBT0030 [16] | |
| WB | Abcam ab6326 [17] | |
| BU-1 | IC | R&D Systems MAB7225 [18] ; Invitrogen MA3-071 [19] ; MilliporeSigma MAB3510 [20] |
| FC | Invitrogen MA3-071 [21] ; MilliporeSigma FCMAB101A4 [22] | |
| IHC-Free | Invitrogen MA3-071 [23] | |
| IHC-P | Invitrogen MA3-071 [24] ; MilliporeSigma 05-633 [25] ; MilliporeSigma MAB3510 [25] | |
| B44 | ChIP | BD Biosciences 347580 [26] |
| IC | BD Biosciences 347580 [4, 27] | |
| FC | BD Biosciences 347580 [28] | |
| IHC-F | BD Biosciences 347580 [29] | |
| IHC-Free | BD Biosciences 347580 [30] | |
| IHC-P | BD Biosciences 347580 [31] | |
| RPPA | BD Biosciences 347580 [7] | |
| MoBu-1 | IC | Invitrogen B35130 [32, 33] ; Invitrogen B35128 [34] ; Invitrogen MA1-19213 [35] ; Santa Cruz Biotechnology sc-51514 [36] ; Abcam ab8039 [37] |
| FC | Invitrogen B35130 [38, 39] ; Invitrogen B35129 [40] ; Invitrogen B35133 [41] | |
| IHC-F | Invitrogen B35128 [42, 43] ; Invitrogen B35130 [44] | |
| IHC-P | Invitrogen B35138 [45, 46] ; Invitrogen MA1-19213 [47] ; Santa Cruz Biotechnology sc-51514 [48] | |
| IHC-Free | Santa Cruz Biotechnology sc-51514 [49] ; Invitrogen MA1-19213 [50] | |
| WB | Invitrogen B35128 [51] | |
| Bu20a | ChIP-Seq | Dako M0744 [52] |
| FC | Invitrogen 11-5071-42 [53], Invitrogen 14-5071-80 [54, 55] ; Invitrogen 14-5071-82 [55] ; Invitrogen MA1-81890 [55] ; BioLegend 339801 [56] ; BioLegend 339807 [57] ; Cell Signaling Technology 5292 [28] ; Dako M0744 [58] | |
| IC | Dako M0744 [59] ; Cell Signaling Technology 5292 [60, 61] | |
| IHC-F | Cell Signaling Technology 5292 [62] | |
| IHC-Free | Dako M0744 [63] | |
| IHC-P | Bio-Rad MCA2483 [64] ; Cell Signaling Technology 5292 [61] ; Dako M0744 [64] | |
| WB | Invitrogen MA1-81890 [65] | |
| BU-33 | ELISA | MilliporeSigma B2531 [66] |
| IC | Invitrogen 03-3900 [67] ; MilliporeSigma B8434 [68] ; MilliporeSigma B2531 [68] | |
| FC | MilliporeSigma B8434 [28, 69] | |
| IHC-F | Invitrogen 03-3900 [70] ; MilliporeSigma B2531 [71] ; MilliporeSigma B8434 [72, 73] | |
| IHC-P | MilliporeSigma B8434 [74] ; MilliporeSigma B2531 [75] | |
| IP | MilliporeSigma B2531 [76] | |
| WB | MilliporeSigma B8434 [77] ; MilliporeSigma B2531 [77] | |
| RIP | MilliporeSigma B2531 [78] | |
| 3D4 | IC | BD Biosciences 555627 [79] ; BD Biosciences 560810 [80] |
| FC | BD Biosciences 555627 [81] ; BD Biosciences 558599 [82] | |
| IHC-F | BD Biosciences 555627 [83] | |
| IHC-Free | BD Biosciences 555627 [84] | |
| IHC-P | BioLegend 364105 [85] ; BD Biosciences 555627 [86] ; BD Biosciences 563445 [87] | |
| ChIP-Seq | BD Biosciences 555627 [88] | |
| WB | BD Biosciences 555627 [89] | |
| IIB5 | IC | MilliporeSigma MAB3222 [90] |
| ICW | MilliporeSigma MAB3222 [91] | |
| IHC-F | Santa Cruz Biotechnology sc-32323 [92] | |
| IHC-P | Abcam ab8955 [93] ; Abcam ab8152 [94] | |
| BMC9318 | IC | MilliporeSigma 11170376001 [95] |
| IHC-Free | MilliporeSigma 11170376001 [96] | |
| IHC-P | MilliporeSigma 1170376001 [97] ; MilliporeSigma 11170376001 [98] | |
| G3G4 | FC | Developmental Studies Hybridoma Bank G3G4 [99] |
| IC | Developmental Studies Hybridoma Bank G3G4 [100, 101] | |
| IHC-F | Developmental Studies Hybridoma Bank G3G4 [102] | |
| IHC-P | Developmental Studies Hybridoma Bank G3G4 [103] | |
| AH4H7-1 / 131-14871 | IC | MilliporeSigma MAB3424 [104] |
| IHC-P | MilliporeSigma MAB3424 [105] | |
| 3H579 | IHC | Santa Cruz Biotechnology sc-70441 [106, 107] |
| BU5.1 | IC | MilliporeSigma CBL187 [80] |
| BU6-4 | FC | Santa Cruz Biotechnology sc-56259 [108] |
Liboska R et al examined the affinities of BMC9318 from Roche, B44 from Becton Dickinson, Bu20a from BioLegend, BU-33 from Sigma-Aldrich, BU6-4 from Genetex, BU5.1 from Millipore, MoBu-1 from Exbio and BU1/75 from Abcam against 2'-deoxy-5-ethynyluridine (EdU)and found that with the exception of MoBu-1 clone, all reacted with Edu [109]. Ligasová A et al evaluated the affinities of anti-bromodeoxyuridine monoclonal antibodies: BMC9318, Bu-33, B44, Bu6-4, Bu20a and Bu5.1, against oligonucleotides with BrdU at 5' or 3' end or in the middle, and found that clones Bu-33 and Bu5.1 had very low affinity with the tested oligonucleotides and all of the tested clones exhibited the highest affinity to the oligonucleotide with BrdU at the 5' end; clones Bu20a, BMC9318 and Bu5.1 clones exhibited the lowest affinity to the oligonucleotide with BrdU at the 3' end; clones Bu-33, B44, and Bu6-4 to the oligonucleotide with BrdU in the central part of the chain [110]. In addition, clones B44 and BMC9318 were found to work with all four protocols: hydrochloric acid (2N and 4N), DNase I and copper ion treatment, for the detection of BrdU in HeLa and HCT116 cells [110]. The authors in a follow-up article attributed the differing affinities of the above clones to their varying speed of dissociation and optimized an enzymatic protocol to achieve higher signal/background ratio, even for those clones with lower affinities [80].
Compared to BrdU antibodies, researchers cite anti-DNA antibodies much less frequently. Table 2 lists the cited DNA antibody clones, their strand specificity, and other information. One active research area is auto-antibodies in diseases such as systemic lupus erythematosus and isolated cases of viral infections. DNA auto-antibodies are found in 100% cases of systemic lupus erythematosus, and also in inbred mouse strains with an auto immune disorder similar to systemic lupus erythematosus. Most of these auto-antibodies react more strongly with denatured than with native DNA [111]. Some discriminate among helical DNAs of differing base sequence [111]. Quite often, these auto-antibodies with high affinity contain arginine, asparagine, lysine, and tyrosine in the CDRs, enabling the formation of electrostatic interactions between positively charged CDRs and negatively charged phosphodiester-deoxyribose backbone and hydrogen bonds between these amino acids and DNA. While review articles such as the one by Hu Z et al [2], list many more clones, most of them are either not available commercially or through non-profit organizations, or not cited in recent literature.
DNA antibodies have been used in a variety of applications. For example, Amini P et al detected NET formation in primary mature mouse neutrophils using monoclonal mouse anti-dsDNA antibody ( MAB1293) from MilliporeSigma [112].
| Specificity | Application | Clone | Supplier, catalog number, and reference |
|---|---|---|---|
| ds/ss | IC | AC-30-10 | Progen [113], MilliporeSigma CBL186 [114] |
| ds | ChIP | AE-2 | MilliporeSigma MAB1293 [115] |
| IC | 35I9 | Abcam ab27156 [116] | |
| AE-2 | MilliporeSigma MAB1293 [112] | ||
| HYB331-01 | Santa Cruz Biotechnology sc-58749 [117] | ||
| ss | FC | 16-19 | MilliporeSigma MAB3034 [118] |
| IC | 16-19 | MilliporeSigma MAB3034 [4, 11] | |
| IHC | F7-26 | MilliporeSigma MAB3299 [119] | |
| 8-OHdG | IC | 15A3 | QED Bioscience 12501 [120, 121] |
| Northwestern blotting | 15A3 | QED Bioscience 12501 [120] | |
| IP | 15A3 | QED Bioscience 12501 [120] | |
| IHC-P | 15A3 | QED Bioscience 12501 [121, 122] | |
| N45.1 | Genox / Nikken SEIL [123] | ||
| BPDE-DNA | IHC-P | 5D11 | Santa Cruz Biotechnology sc-52625 [124] |
| cisplatin DNA adducts | IHC-P | clone CP9/19, Abcam | MilliporeSigma MABE416 [126] |
| WB | 1CR4 | MilliporeSigma MABE416 [127] |
Scheer U et al immunized BALB/c mouse with cytoskeletal preparations, fused the spleen cells with mouse myeloma line Ag 8.653, and screened for clones with IHC on frozen rat and mouse liver sections [128]. The resultant clone, with IgM subclass, may have resulted from the DNA present in the immunogen or from an autoantibody producing spleen cell [128]. AC-30-10 binds to both double and single-stranded DNA and has no cross-reactivity with deoxyribonucleotides and RNA [128].
Frankfurt OS obtained monoclonal antibody F7-26 from a mouse immunized with DNA treated by nitrogen mustard (HN2) [129]. The clone, of IgM subclass, binds to ssDNA, but not to dsDNA or RNA [2].
Sarek G et al stained mouse ear fibroblasts with the anti-ssDNA antibody (MilliporeSigma, MAB3034) in order to ascertain no broken DNA tracks in DNA combing [4].
8-OHdG (8-hydroxy-2'-deoxyguanosine) results from DNA oxidative damage by hydroxy radical, singlet oxygen and direct photodynamic action. 8-OHdG antibody clone N45.1 from Genox / Nikken SEIL detects 8-OHdG in tissues, sera, urine and other biomaterials with IHC and ELISA. It does not cross react with RNA oxidation products such as 8-hydroxy-guanine and 8-hydroxy-guanosine. Aoki A et al used 8-OHdG staining by clone N45.1 as an oxidative stress marker in mouse placentas [123].
One of the commonly used RNA antibody clones is Y10b. Lerner EA et al fused spleen cells from an unimmunized female MRL/1 mouse with MRL/l mice were bred and maintained myeloma SP 2/0 and screened to obtain the clone Y10b, of the IgG2a class, which is specific to rRNA [130, 131].
| Specificity | Application | Clone | Supplier, catalog number, and reference |
|---|---|---|---|
| rRNA | IC | Y10b | Novus Biologicals NB100-662 [132] |
| Y10b | Invitrogen MA1-16628 [133] | ||
| IHC-Free | Y10b | Invitrogen MA5-16064 [134] | |
| IHC-P | Y10b | Novus Biologicals NB100-662 [135] | |
| IP | Y10b | Novus Biologicals NB100-662 [136] |
| Application | Clone | Supplier, catalog number, and reference |
|---|---|---|
| Dot blot | S9.6 | MilliporeSigma MABE1095 [137] ; KeraFAST ENH001 [138] |
| ChIP / DRIP | S9.6 | KeraFAST ENH001 [139] ; MilliporeSigma MABE1095 [137] |
| IC | S9.6 | MilliporeSigma MABE1095 [137] ; KeraFAST ENH001 [138] |
| Immuno-EM | S9.6 | KeraFAST ENH001 [140] |
| WB | S9.6 | KeraFAST ENH001 [139] |
Boguslawski SJ et al immunized BALB/c mice with a DNA:RNA hybrid preparation combined with methylated thyroglobulin and fused mouse spleen celles with myeloma cells (Sp2/0-Ag14). The selected clone, S9.6, is of IgG2a subclass with kappa light chains, and has a high affinity for DNA:RNA duplex (Kd = 1.18 x 10-11 M), and 1/1000 or less affinity to single- or double-stranded DNA or RNA [141]. S9.6 antibody has been used by, for example, Lambo S et al [137] and NK Karanam et al [138] to study R-loops, and Jiang YF et al to indicate compromised mtDNA replication in flies [140].
- Gonchoroff N, Greipp P, Kyle R, Katzmann J. A monoclonal antibody reactive with 5-bromo-2-deoxyuridine that does not require DNA denaturation. Cytometry. 1985;6:506-12 pubmed
- Bray K, Gillette M, Young J, Loughran E, Hwang M, Sears J, et al. Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res. 2013;15:R91 pubmed
- Pierzyńska Mach A, Szczurek A, Cella Zanacchi F, Pennacchietti F, Drukała J, Diaspro A, et al. Subnuclear localization, rates and effectiveness of UVC-induced unscheduled DNA synthesis visualized by fluorescence widefield, confocal and super-resolution microscopy. Cell Cycle. 2016;15:1156-67 pubmed publisher
- Li Q, Guo Y, Chen F, Liu J, Jin P. Stromal cell-derived factor-1 promotes human adipose tissue-derived stem cell survival and chronic wound healing. Exp Ther Med. 2016;12:45-50 pubmed
- Allameh A, Jazayeri M, Adelipour M. In Vivo Vascularization of Endothelial Cells Derived from Bone Marrow Mesenchymal Stem Cells in SCID Mouse Model. Cell J. 2016;18:179-88 pubmed
- Vergaño Vera E, Diaz Guerra E, Rodríguez Traver E, Méndez Gómez H, Solis O, Pignatelli J, et al. Nurr1 blocks the mitogenic effect of FGF-2 and EGF, inducing olfactory bulb neural stem cells to adopt dopaminergic and dopaminergic-GABAergic neuronal phenotypes. Dev Neurobiol. 2015;75:823-41 pubmed publisher
- Stollar B, Zon G, Pastor R. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc Natl Acad Sci U S A. 1986;83:4469-73 pubmed
- Scheer U, Messner K, Hazan R, Raska I, Hansmann P, Falk H, et al. High sensitivity immunolocalization of double and single-stranded DNA by a monoclonal antibody. Eur J Cell Biol. 1987;43:358-71 pubmed
- Frankfurt O. Detection of DNA damage in individual cells by flow cytometric analysis using anti-DNA monoclonal antibody. Exp Cell Res. 1987;170:369-80 pubmed
- Lerner E, Lerner M, Janeway C, Steitz J. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981;78:2737-41 pubmed
- Hyson R, Rubel E. Activity-dependent regulation of a ribosomal RNA epitope in the chick cochlear nucleus. Brain Res. 1995;672:196-204 pubmed
- Zheng J, Kelly T, Chang B, Ryazantsev S, Rajasekaran A, Martin K, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291-303 pubmed
- Boguslawski S, Smith D, Michalak M, Mickelson K, Yehle C, Patterson W, et al. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods. 1986;89:123-30 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
