Bats are the natural host for a variety of dangerous and often lethal viral agents, such as Hendra, Nipah, Marburg, Ebola and the coronavirus that is the etiological agent of the coronavirus disease 2019 (COVID-19). The adaptive evolution of the bats resulted in a balance between host defense and tolerance, which allows them to co-exist with viruses that are highly pathogenic in humans. Other interesting findings about the biology of the bats are the low rate of cancer incidence and the slow advancement of the ageing process. This article provides an overview on the approaches for the use of bats as animal models for the identification of the physiological factors that render apathogenic deadly viruses and for the analysis of the low occurrence of tumors and the slow progression of ageing.
A number of outbreaks of viral-related diseases have been reported in the last decades in different parts of the world, such as the hemorrhagic fevers induced by Marburg and Ebola viruses [1, 2], fatal respiratory or neurological illnesses that are associated with the Hendra virus infection [3], acute respiratory infection and dangerous encephalitis that may derive from the exposure to the Nipah virus [4] and the coronaviruses [5, 6], which have been responsible for the severe acute respiratory syndrome (SARS) in China in February 2003 [7], the Middle East respiratory syndrome (MERS) in Saudi Arabia in 2012 and for the current pandemic of the coronavirus disease 2019 (COVID-19) [8]. The initial COVID-19 cases were reported from Wuhan, China and it rapidly spread to the rest of the World. Covid-19 has caused more than 3 million deaths worldwide, as of April 16th, 2021.
Epidemiological studies indicated that all the above referenced outbreaks were associated with the zoonotic transmission of bat-borne viruses. Studies on the biology of the bats might shed useful insights on the pathogenicity of potentially deadly viruses in humans and, eventually, lead to the development of novel therapeutic approaches for the treatment of harmful viral diseases, along with the prevention of possible future zoonotic spillovers.
Bats are placental mammals that belong to the order of Chiroptera, which, from the Ancient Greek, stands for hand-wing (χείρ – cheir = hand; πτερόν – pteron = wing), as the upper forelimbs were adapted through evolution as wings for the flight. Bats have undergone 64 million years of adaptive evolution and constitute more than 20% of the currently existing mammalian species [9]. So far, 1,423 types of bats have been classified, whereas the overall number of known species of mammals is in the range of 6,400. These unique flying mammals are widely distributed around the world, with the exception of some oceanic islands and regions with extreme climate conditions, such as deserts and, naturally, the Arctic and Antarctic Poles.
Bats roost in caves, crevices, hollowed trees, foliage, barns, bridges and even houses and various types of other buildings and they have a fundamental role for a variety of biological functions, which comprise seed dispersal and pollination, fertilization and in keeping insect populations under control.
Some bat species are homothermic, whereas others are heterothermic. Bats can either utilize hibernation, or a diurnal sporadic torpor to save energy [10] and feed on a wide variety of different types of diets, such as insects, nectar, pollen, fruit, fish and blood, as in the case of Desmodus rotundus that is commonly termed bat vampire. Microbats in particular have the sensing powers of echolocation and magnetoreception that allows them to distinguish the polar north from the south [11-14].
Aerial transport constitutes an advantage; however, flying is associated with the high consumption of energy [15]. Bats have metabolic rates when they fly that can be in the range of 2.5 to 3 times higher than the metabolic rates of similar size terrestrial mammals [16]. The high demand of energy causes the depletion of 50% stored metabolic energy in a single day. Flying bats utilize 1200 calories per hour [17-19] and, for this reason, they must have a variety of metabolic adaptation and efficient airflow systems during the flight, in order to cope with the high demand of energy, which would otherwise inevitably result in starvation, with consequent death [20]. For instance, their heart rate increases 4 to 5 times and reaches 1,066 beats per minute during flight [19]. Cyclic bradycardic state is a key factor in optimizing the energy conservation by 10% in non-flying times, which can counteract the effects of high heart rates that take place during flight [19].
Interestingly, regardless of the high metabolic rates and small body sizes, bats exhibit lifespans that can be 3.5 higher than non-flying mammals with a comparable body mass [21, 22]. Six types of bats can live for three decades in the wild: Plecotus auratus, Myotis lucifugus, Myotis brandti, Myotis blythii, Rhinolophus ferrumequinum and Pteropus giganteus [23]. One hypothesis for the longevity of bats is related to their hibernation process, during which the metabolic rate is slowed down. It has been observed that hibernating bats live longer than non-hibernating ones [24, 25].
Additional studies are ongoing for the analysis of microRNA molecules in the delay of the effects of ageing in cells and/or tissues of the bats [26], whereas previous reports showed that telomeres do not get shorter with the progression of age in bats, which could be a further mechanism in contrasting the effects of ageing [27, 28].
The low incidence of cancer is another very interesting biological characteristic of the bats [26, 29]. A recent study demonstrated that bat-derived cells are more resistant to genotoxicity than human and mouse cells [29]. The ABC transporter ABCB1 is expressed in considerably high levels in bat cells and removes toxic substances that may ultimately damage the DNA genome [29].
Other important factors that are involved in the low incidence of cancer in bats are related to the expression of particular kinds of microRNA molecules. Studies have shown that microRNAs that act in the context of tumor suppression are upregulated with the progression of age in bats, whereas microRNAs that might promote carcinogenesis are downregulated [26, 30, 31]. Interestingly, the ageing process induces the opposite effects in all other mammalian species, in which the incidence of malignancies increases with age [26, 30, 31].
A remarkable finding about the immunology of the bats is the limited degree of inflammations, when the host immune system responds to infectious agents. Inflammatory responses might otherwise result in the onset of various pathological conditions in humans. The ability of the bats to limit inflammatory reactions to fight infections contributes to the slowing-down of the ageing progression and to the reduction of ageing-related illnesses [32], including cancer [33-36]. For instance, in humans and mice, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) functions as an intracellular sensor for a broad range of microbial components, endogenous danger signals that trigger inflammation and various environmental irritants. All these factors stimulate the NLRP3 inflammasome, which, in turn, induces the expression of pro-inflammatory cytokines IL-1β and IL-18, along with the gasdermin D-mediated pyroptotic cell death gene [37-39]. The NLRP3 inflammasome may also be implicated in acute myocardial infarction [40]. In contrast to human and murine systems, bats exhibit low levels of NLRP3 activities, even in the presence of high viral loads, as indicated by studies on three distinct RNA viruses: the influenza virus, coronavirus and Melaka virus [32]. Bat apoptosis-associated speck-like protein (ASC2) was shown to function as a negative regulator of inflammasomes [41]. In particular, bat ASC2 suppressed inflammation caused by multiple viruses and decreased mortality of influenza A virus infection. It also interfered with SARS-CoV-2-immune-complex-induced activation of the inflammasome. Furthermore, transgenic expression of bat ASC2 in mice decreased the activity of experimentally induced peritonitis. Therefore, the avoidance of acute inflammatory reactions is likely to enable the coexistence and survival of bats to viral infections, which, however, render these animals suitable hosts for viruses like coronaviruses, Hendra virus, Nipah virus, Marburg and Ebola viruses.
Coronaviruses have caused the outbreaks of the severe acute respiratory syndrome (SARS) in China in February 2003, the Middle East respiratory syndrome (MERS) epidemic that was reported in Saudi Arabia in 2012 and the recent pandemic of the coronavirus disease 2019 (COVID-19).
Coronaviruses are enveloped single stranded positive sense RNA viruses with a nucleocapsid of helical symmetry and belong to the Orthocoronavirinae subfamily of the Coronaviridae family, which is in the order of Nidovirales [42, 43].
The size of the genome of the coronaviruses may vary from 26 to 32 kb and, therefore, they are the RNA viruses with the largest genome [44]. Club-like spikes protrude from the round-shaped envelope and confer an electron micrograph imaging that resembles the shape of the solar corona, or halo, which provided the typical name coronavirus [45]. Corona is the Latin term for crown. In turn, the Latin word corona derived from the ancient Greek korone. Until 2020, forty-five types of coronaviruses have been identified.
Coronaviruses cause illnesses in humans, birds, cows, pigs and mice. Coronavirus infections mainly affect the respiratory and gastrointestinal tract in humans, whereas only respiratory tract infections have been reported in birds. Human illnesses may vary from the symptoms of the so-called common cold to more severe pathological conditions, which may be lethal, such as in the cases of SARS, MERS and Covid-19. Coronavirus infections set off diarrhea in pigs and cows [46, 47], whereas they induce hepatitis and encephalomyelitis in mice [48, 49].
As anticipated, coronaviruses target the epithelial cells of the respiratory and gastrointestinal tract in humans and they are mainly internalized in form of aerosols, or droplets. However, coronaviruses can also infect humans via fomite, or through an oral-fecal route.
The spikes of the coronaviruses bind to the angiotensin-converting enzyme 2 (ACE2) receptor of the epithelial cells of the respiratory tract in humans [50]. Studies in pigs showed that the transmissible gastroenteritis coronavirus utilizes the alanine aminopeptidase (APN) receptor [51]. The same receptor is utilized by the coronaviruses to infect the epithelial cells of the gastrointestinal tract in humans [52]. Each coronavirus particle carries on average seventy-four spikes on the envelope [53]. The spikes are 20 nm long and consist of an S protein trimer. The S protein contains an S1 and S2 subunits. The resulting trimeric S protein belongs to the class I fusion protein, which allows for the binding to the cellular receptor, which is followed by the membrane fusion between the viral envelope and the cell membrane. The receptor binding domain (RBD) is situated in the subunit S1 on the head of the spike, whereas the S2 subunit constitutes the stem that holds the spike of the viral surface and, following the protease activation, allows for the fusion process of the viral and cellular membranes. The S1 and S2 subunits are not covalently bound [53].
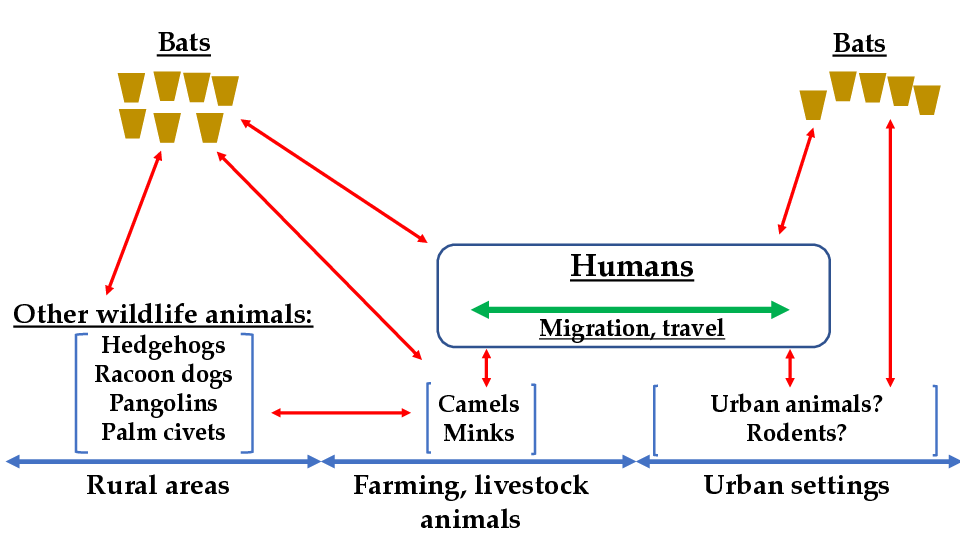
The natural form of transmission of the etiological agent that causes Covid-19 took place initially in rural areas, starting from bats and affecting other wildlife animals. Subsequently, the virus infected farming and livestock animals and, lastly, humans (Figure 1). For instance, camels mediated the transmission of MERS-CoV to humans in Saudi Arabia [54]. Minks are other farming animals that acquired the coronavirus that causes Covid-19 [54]. Humans, in turn, brought the infectious agent from the rural areas to urban settings and then the coronavirus was transmitted to urban animals, including local bats (Figure 1). The human population that was initially affected by the coronavirus infection comprised guano farmers and residents of areas that comprise the natural habitats of the bats. Human migration was ultimately responsible for the transmission of the Covid-19 etiological agent to the urban settings populations (Figure 1).
The effects of longitudinal changes in bat species assemblages on the mechanisms of the infections caused by coronaviruses (CoVs) in cave-dwelling bats captured in Ghana have recently been investigated [55]. The study reported different CoV infection patterns between closely related species. Furthermore, the alpha-CoV 229E-like and SARS-related beta-CoV 2b emerged as multi-host pathogens. Overall, bat species showed different CoV competence and highly competent species were observed more frequently in less diverse communities.
To evaluate the diversity of viruses in bats, another study analyzed the mammal-associated viruses using an unbiased meta-transcriptomics approach [56]. The authors reported a frequent simultaneous infection of bats by multiple viral species and spillover, which may promote virus recombination. Moreover, five pathogenic viral species were detected. The detected viruses included a new recombinant SARS-like coronavirus, which is related to both SARS-CoV and SARS-CoV-2 and can bind the human angiotensin-converting enzyme 2 receptor.
A Jamaican fruit bat (JFB, Artibeus jamaicensis) intestinal organoid model of SARS-CoV-2 infection was used to study the mechanisms of antiviral responses in bats [57]. The results demonstrated that infection with SARS-CoV-2 increased viral RNA. Also, SARS-CoV-2 enhanced expression of type I interferons and inflammatory cytokines and stimulated growth of JFB organoids. In line with these results, proteomics showed activated inflammatory signaling, cell repair and SARS-CoV-2 infection pathways.
Ebola and Marburg viruses belong to the Filoviridae family in the order Mononegavirales and have a linear, non-segmented, single-strand negative RNA genome [58]. These two viruses are characterized by high virulence in humans and induce an often-fatal hemorrhagic fever. Ebola virus was initially identified in 1976 [58]. Since then, 20 known outbreaks of Ebola disease have been reported in Africa. In most cases, the outbreaks occurred in rural areas, whereas the outbreak in 2000 affected a semi-urban part of Uganda. A large Ebola virus outbreak occurred in West Africa from 2013 until 2016 and involved both rural and urban regions of three Countries: Guinea, Sierra Leone and Liberia [59-62]. The official reports indicated at least 28,000 cases of Ebola disease, with more than 11,000 deaths. The lethality rate among the reported cases of infection was 62.9% [58].
The most recent Ebola virus outbreaks were observed in May 2018 and in August 2018 in the Democratic Republic of Congo [63, 64]. The May 2018 outbreak approached the city of Mbandaka, which is essential for the transportation system along the Congo river. Fortunately, the outbreak was rapidly contained and only 54 cases of infections with 33 deaths, were reported [63, 64].
The Marburg virus was initially identified in 1967, following hemorrhagic fever outbreaks that took place simultaneously in research laboratories based in Marburg and Frankfurt in Germany and in Belgrade in the former Republic of Yugoslavia, which is currently Serbia. In total, thirty-two people were infected by the Marburg virus and seven died. The outbreak started from imported African green monkeys. The Marburg virus was transmitted to some investigators inside the research facilities and then the virus was passed to family members and to medical personnel. Subsequent studies showed that the natural host for the Marburg virus was the African fruit bat, Rousettus aegyptiacus [65-67].
The analysis of the Rousettus aegyptiacus genome was instrumental in understanding the antiviral immunity of the African fruit bats, which exhibited drastic differences from all other mammal species in terms of genetic arrangements encoding for particular kinds of natural killer cell receptors, MHC class I genes and type I interferons [68]. Basically, the Rousettus aegyptiacus evolution conferred an immune tolerance to viruses. This unique strategy allows bats to coexist with viruses that are highly pathogenic in other mammals and, especially in humans [68].
The Ebola virus contagion among people may happen via direct contact with the blood, or other bodily fluids of infected individuals [69, 70]. Other types of bodily fluids that may carry the virus comprise mucus, saliva, tears, breast milk, feces, urine and semen. So far, there are no reports indicating that the Ebola virus may be transmitted through sweat. The most common route of transmission among humans is via blood, vomit and feces. Ebola virus can infect an individual through the mouth, nose, eyes, abrasions and wounds. Other types of animals that may contract the Ebola virus from the African fruit bats include chimpanzees, gorillas, baboons and duikers, which are a type of antelopes. All these animals may acquire the Ebola virus by eating fruits that were previously bitten by infected African fruit bats [71].
Ebola virus, along with other filoviruses, exhibit high replication rates in several cell types, such as macrophages, monocytes, dendritic cells, hepatocytes, adrenal gland cells and fibroblasts [72]. The Ebola virus replication induces high levels of inflammatory responses, leading to a septic state [73]. The hemorrhagic symptoms appear following the Ebola virus infection of endothelial cells, which occurs within three days after the primary infection of an individual [74].
The membrane of the Ebola and Marburg viruses carries a glycoprotein (GP), which contains two subunits, termed GP1 and GP2. The GP1/2 complex mediates the viral entry into target cells, whereas the soluble GP has a variety of functions, including the inactivation of neutrophils and disfunctions of the vascular system. The soluble GP inactivates neutrophils by binding the CD16b receptor, which, in turn, induces apoptosis in lymphocytes. GP1/2 complex binds C-type lectins, such as L-SIGN, DC-SIGN and hMGL, which are expressed on the membrane of macrophages, monocytes and dendritic cells [75-78]. Another cellular receptor for the Ebola virus is the phosphatidylserine receptor T cell immunoglobulin mucin domain-1 (TIM-1), which is present on the membrane of T lymphocytes [79]. Following the binding to the cellular receptor, the Ebola and Marburg viruses are internalized via endocytosis into the endosome, where the viral membrane fuses with the membrane of the endosome membranes, causing the release of the virion into the cytoplasm [80].
A recent study reported that although 8 of 17 known Marburg virus disease outbreaks in sub-Saharan Africa have been associated with human encroachment on Egyptian rousette bats (ERB), no connection was established for the other 9 outbreaks [81]. Micro-global positioning systems were applied to identify nightly ERB locations. The study showed that Marburg virus-infected ERBs migrate long distances to feed in cultivated fruit trees near human settlements. Thus, the results indicated that ERB foraging behavior may be associated with a Marburg virus spillover risk to humans and can explain the mechanisms of some Marburg virus disease outbreaks.
The Hendra and Nipah viruses are enveloped RNA viruses and belong to the genus Henipavirus, in the order Mononegavirales of the family Paramyxoviridae [82].
Sporadic cases of Hendra virus infections were reported among horses between 1994 and 2010 in Brisbane, a suburb of Hendra, in Australia. Rare cases of infections in humans were also reported from 1994 until 2013. The Hendra virus was transmitted to horses by flying foxes, which belong to the genus of megabats [82].
Infected horses develop a strong febrile respiratory illness, often leading either to the death of the animal, or to euthanasia. Two persons that were in contact with the infected animals contracted a flu-like disease. One of them died as a result of an acute severe interstitial pneumonia [82]. The Hendra virus was isolated from a kidney of the deceased patient and characterized. Zoonotic Hendra virus transmission to humans can also cause fatal encephalitis and multi-organ failure. The rate of fatalities among infected humans was 57% [82].
Nipah virus was identified in 1999, when an outbreak affected pigs and humans in Malaysia and Singapore. Almost 300 people were infected and more than 100 patients died. Studies confirmed that the Nipah virus was transmitted by fruit bats [83, 84]. Subsequent Nipah virus outbreaks affected the Indo-Bangladesh regions [85]. Nipah virus infection causes fever, encephalitis and/or respiratory distress in patients [85]. The fatality rate of the Nipah virus has been estimated at 40% to 75% [4].
In a recent study, bat, rodent and shrew samples obtained from Belgium were tested for orthoparamyxovirus using nested reverse transcription-polymerase chain reaction assays and nanopore sequencing [86]. The authors described the genomes of six new viral species that belonged to Jeilongvirus and Henipavirus. Also, the phylogenetic analysis of jeilongviruses and henipaviruses showed a division of both groups into two clades, one containing bat-borne viruses and the other consisting of rodent- and shrew-borne viruses.
| Types of bat | Food | Links |
|---|---|---|
| Flying fox | Fruit | youtube |
| Silver-haired bat | Mealworm | youtube |
| Orphaned baby bat | Formula milk | youtube |
| Baby bat | Formula milk | youtube |
| Captive-born vampire bat | Blood | SocialBat |
Bats care in research settings is very time consuming and requires sensitivity and dedication from investigators and animal facility technicians. Bats must be able to adapt to the conditions of the artificial situation of a research setting. In their natural environment, most bats procure the food in flight. Obviously, this is no longer possible in a research facility, in which the animals must be individually trained in eating mealworms (Tenebrio), or other types of food from a Petri dish. Each bat may require up to 30 minutes for the initial training feeding period. In some cases, bats learn how to feed independently in three days, whereas in other cases it may take up to four weeks for the animals to learn how to feed themselves from a dish. The links of some videos showing feeding techniques for bats are listed in Table 1. Naturally, the feeding training in a research setting, or in a zoo must take place in the evening, as bats maintain their nocturnal pattern in captivity. It has been estimated that each big brown bat can eat up to 60 mealworms per night. Therefore, large mealworm cultures are required. Other types of bats consume a big quantity of fruit, whereas the co-called vampire bats require blood (Table 1).
Bats can be captured from their roosts in the summer, or from the hibernacula in the winter season. Licenses or permits are required in the majority of countries for fieldwork, capturing and ultimately transportation of animals. In addition, the Centers for Disease Control and Prevention (CDC) has warned about the risk of the transmission of rabies from bats to humans and/or to other animals and has therefore recommended to avoid direct physical contact and to keep the bats in quarantine for a period of three months, which is the standard incubation period for rabies [87, 88]. The quarantined animals have to be monitored by specialized personnels to detect the onset of rabies-related symptoms. Bats must be also inspected for the presence of ectoparasites and fungal infections. Naturally, another important concern is related to the possible transmission of coronaviruses to humans. Studies conducted in the summer of 2006 and 2007 detected group 1 coronaviruses in bats in North America [89] and in Canada [90], respectively.
It is necessary to take all precautions that are required to avoid harm to bats, when they are caught and transported to research facilities. Adult female bats should be captured in the autumn, or winter. If the animals are caught in the summer, the investigators must make sure to release lactating females, to prevent the death by starvation of young bats. Signs of lactation in females can be easily identified by enlarged mammary glands and by the presence of bare skin around the nipples. Capturing hibernating bats can be easily carried out in the winter season. Thousands of hibernating bats can be found in caves and they can be simply taken manually from their hanging position. However, preventive measures must be adopted to minimize as much as possible the awakening of other hibernating bats, which is a process that requires energy. If the animals are disturbed frequently, they will deplete a lot of energy, in a period when the food is not available, which, in turn, may result in death of the animals because of starvation. For this reason, caves should not be visited more than once, or, eventually, twice.
| Type of trapping device | Notes for the trapping device | Links |
|---|---|---|
| Mist nets | How to set up a mist net | youtube |
| How to remove a bat from a mist net | youtube | |
| Harp trap | How to set up a harp trap | youtube |
| Using harp traps to capture bats | youtube youtube | |
| Wire-mesh cylinder with a smooth, fairly wide metal rim at the upper border | Link to the document: | CCAC |
During the non-hibernating seasons, bats can be captured with various devices and different strategies. One of the first devices that was developed to capture bats consists of a wire-mesh cylinder with a smooth metal rim situated at the upper border [91]. Other trapping devices are based on mist nets to catch bats as they emerge from a roost [89] and harp traps set up at the opening of a cave during a mating swarm [90]. Table 2 reports the links to the videos, or the descriptions of various trapping devices for the harmless capture of bats.
Motor vehicle transportation of captured bats requires a narrow mesh cage, with a handle on a sliding cover, which can be locked [92]. The wire mesh cage has to be tightly positioned within a wooden box with slits, so the air can circulate. The base of the cage consists of a metal tray, which holds a wet foam rubber sheet for thirsty bats [92].
The rooms for the housing of bats cannot have gaps, through which the animals might eventually escape. Small size bats have the ability to squeeze themselves into openings that are only 1 centimeter wide. This is the typical space that can be found in the perimeter of ventilation shafts screens and drainage systems, or the gap between the floor and the bottom part of a door. Fine wire mesh screens can be utilized to seal off various openings, whereas the bottom part of doors require either rubber, or flexible plastic sheets [93].
Long-term captivity requires different housing conditions for non-hibernating and hibernating bats. Housing is certainly easier for non-hibernating bats, as they only need a standard room that is usually utilized to quarantine the animals after the capture. The temperature of the room for the day and night has to be set on a biweekly basis, in order to mimic the typical outdoor conditions. The same goes for the photoperiod, which has to be adapted to outdoor conditions. The intensity of the light must be kept low in the environment. If there is a window, the room does not need illumination. The humidity of the room is not a critical factor during the non-hibernating period. The typical bat cage for non-hibernating animals is a walk-in wire mesh and is sufficiently large to allow for short flights. Obviously, the door has to be efficiently closed. The dimensions of the bat cage are in the range of 3 x 1 x 2 meters. Smaller cages covered with either a towel, or dark plastic are placed on shelves inside the bat cage. Bats require this type of setting for hiding [93]. It is important that bats are allowed to fly for half an hour inside the holding room, before feeding each day. Urine and feces of the bats will be dropped on paper towels that are present at the bottom of the cages. In this way, it will be easier to remove the waste, which will be then incinerated. It is sufficient to clean the cages once a month, unless the feces are dropped on the wire mesh. In this case, the cages must be cleaned more frequently [93].
Hibernating bats require a dark, humid cold room set at 5C in the winter season, in order to stimulate the hibernation process [93]. The animals must accumulate up to 50% of fat of the body weight, prior to hibernation. The average amount of fat that is utilized by each animal during the state of hibernation is in the range of 1 gram per month. Hibernating bats can be placed in cold rooms inside mouse cages with a wire mesh cover. The typical dimensions of mouse cages are 28 x 20 x 15 centimeters. Not more than three bats can be accommodated inside the same mouse cage. The humidity is an important factor for bats during the hibernation period. In order to keep high humidity, it is necessary to place a stack of 25 wet folded paper towels into the mouse cages. A pad composed of plastic and many layers of cotton has to be placed around the inner walls of the mouse cage and must be in contact with the wet paper towels in the bottom. The cover will keep the pad in the proper position, inside the mouse cage. A coarse dish towel has to be placed underneath the cover, to leave a small space to allow for the circulation of the air. Another stack of wet paper towels is then placed on top of the lid and wrapped with a plastic sheet, leaving a narrow airspace.
A Petri dish with water and one with mealworm larvae must be present in each cage, as bats eventually arouse from hibernation and feel the need to drink, eat, urinate, defecate and re-hibernate. If the experiment requires the removal of bats during the hibernation period, each animal must be alone in the cage, so other hibernating bats will not be disturbed. The cages must be regularly checked every six to eight weeks to restock mealworms and remoisten paper towels.
Breeding of bats in captivity is not practical and it is not recommended. Bats are monestrous animals and their mating in a research setting would require an extensive care. Pregnant females should be collected from the hibernacula, toward the end of the hibernating season. The mating takes place in the autumn and the females store the sperm in the genital tract until the end of the hibernation, when the ovulation and eventually the fertilization occur. The gestation period is comprised between eight to ten weeks. Each pregnant female gives birth to one or two young.
In research facilities, bats can be easily picked up by manual restraint, when the animals are either in the hanging, or sitting position [94]. The wings are immobilized with a gentle pressure from the thumb and the middle finger of the writing hand, while the index finger is pointed to the caudal head region of the animal. The fingers of the opposite hand slide forward under the belly to reach and close the jaw from below, when the animal is gently pulled by the writing hand. At this stage, the bat cannot bite and has to release the claws, as a result of the gentle pull. Following the release of the claws, the bat places the legs on the hand of the operator, which is now bent to enfold the animal. At this juncture, the thumb and the middle finger can be removed from the wings, without squeezing the bat, which is now in a relaxing position [94]. If required by the protocol, the bat can be immobilized on top of a cotton pad placed on the table, by holding the head and the wings, as already described. In the presence of bright light, the eyes of the animal must be loosely covered with a towel, to prevent agitation [94].
The use of anesthetics in bats may be avoided with the use of hypothermia. The animals are placed overnight in a cold room set at 10 C. Vein punctures and other nonpainful procedures may be conducted on animals in a cold-induced torpid state. Painful surgical techniques require the use of anesthetics, in addition to hypothermia. Anesthesia can be administered with an intraperitoneal injection of pentobarbital sodium at doses ranging from 40 to 60 mg per kg of body weight, to induce either light or deep sleep, respectively. The room has to be noise free and the animals cannot be touched until the anesthetic is effective, which may require up to 15 minutes. Following the administration of the anesthetic, the animals must be placed in plastic mouse cages with paper towels in the bottom. Light cotton cloth is used to cover loosely the anesthetized bats. The effect of the anesthetic wears down roughly one-hour post-injection. The recovery time is approximately one hour at a temperature of 23 C, or more than three hours at 20 C. Animals must have access to water placed in a Petri dish. However, the bats must fast overnight, in order to prevent vomiting.
Blood collection from bats requires a stereoscopic microscope for the puncturing of the blood vessels. The total volume of blood that can be collected is in the range of 10% of the lean body weight of a brown bat. A cotton pad must be placed underneath the bat, which has to be gently restrained with a gloved hand, with the fingers slightly bent [94]. The torpid bat settles in this position and falls asleep. The thumb of the restraining hand is moved aside, while the other hand spreads the wing with caution and holds it down gently against the cotton pad with the index finger. A light source pointed at the wing will show the vessels and intensify the circulation of the blood. A hypodermic needle can be utilized to puncture a small vessel and to collect the blood sample.
A flexible cannula is required for the collection of blood from arteries or veins. A 4 cm PE 10 polyethylene tubing is attached to a 30-gauge hypodermic needle, lacking a luer lock hub, whereas the other end of the tubing is connected to a Tuohy-Borst Adapter, which, in turn, is inserted into a hypodermic syringe [94]. If large quantities of blood are required, the bat has to be euthanized by cervical dislocation [95], the thoracic cavity must be immediately opened and the blood is withdrawn from the heart. In addition to cervical dislocation, bats can be euthanized by decapitation, or by an overdose of anesthetic.
Urine samples can be obtained from bats in the awakening period, which is the normal time when bats urinate. Therefore, the operators must remove the bats from the cage while they are still sleeping, hold them in an upright position and place a small glass vial under the eternal urethral opening. The vial has to be encased by masking tape to provide support to the hind legs of the bats. Fecal samples can be easily taken from the paper towels that have been placed in the bottom of the cage. For fresh feces samples, the collection has to be carried out as the animals feed, because bats frequently defecate while they eat.
Some bat-derived cell lines are currently available from the American Tissue Culture Collection (ATCC) (10801 University Boulevard, Manassas, VA 20110 USA) and Millipore Sigma (PO Box 14508, St. Louis, MO 63178, USA) (Table 3).
| Organism, age and gender | Tissue | Morphology | Culture properties | Culture medium | Company | Catalog number |
|---|---|---|---|---|---|---|
| Tadarida brasiliensis, bat, free-tailed. Adult, female | Lung | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium, 10% heat-inactivated fetal bovine serum and 1% L-glutamine (2 mM stock solution) | ATCC | Tb 1 Lu (ATCC® CCL-88™) |
| Tadarida brasiliensis, bat, free-tailed. Adult, female | Lung | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium, 10% heat-inactivated fetal bovine serum and 1% L-glutamine (2 mM stock solution) | ATCC | ATCC® CRL6564™ |
| Myotis velifer incautus, bat, mouse -eared. Adult, female | Skin-derived tumor | Epithelial tumor | Adherent. | Dulbecco's Modified Eagle's Medium, 10% heat-inactivated fetal bovine serum and 1% L-glutamine (2 mM stock solution) | ATCC | ATCC® CRL6011™ |
| Myotis velifer incautus, bat, mouse-eared. Adult, female | Interscapular tumor | Epithelial tumor | Adherent | Dulbecco's Modified Eagle's Medium, 10% heat-inactivated fetal bovine serum and 1% L-glutamine (2 mM stock solution) | ATCC | ATCC® CRL6012™ |
| Macrotus waterhousi californicus, bat, mouse-eared. Age and gender not specified | Unknown. Possibly heart | This cell line is neither produced nor fully characterized by ATCC. | Not specified. | Dulbecco's Modified Eagle's Medium, 10% heat-inactivated fetal bovine serum and 1% L-glutamine (2 mM stock solution) | ATCC | ATCC® CRL6013™ |
| Original organism not specified. Adult, female | Lung. | Epithelial | Adherent | EMEM (EBSS), + 10% Fetal Bovine Serum (FBS), 1% L-Glutamine (2 mM stock solution), 1% Non-essential amino acids (NEAA) | Millipore Sigma | TB1 Lu (NBL-12) |
Several other bat-derived cell lines have been produced by various research laboratories (Table 4) [96-99]. Bat fetuses can also be utilized for the production of cell lines and they might be available from local zoos (Table 4) [98].
| Organism, age and gender | Tissue | Cell line name | Morphology | Culture properties | Culture medium | Title and bibliographic reference (PMID) |
|---|---|---|---|---|---|---|
| Pipistrellus ceylonicus. (Vespertilionidae). Embryo. Female | Embryonic | NIV-BtEPC | Heterogenous | Adherent | Dulbecco's Modified Eagle's Medium (DMEM), with 10% heat inactivated fetal bovine serum and 1% L-glutamine (200 mM stock solution). | Establishment of cell line from embryonic tissue of Pipistrellus ceylonicus bat species from India & its susceptibility to different viruses [96] |
| Perimyotis subflavus (tricolored bat). Adult. Gender not specified | Lung | PESU-B5L | Epithelial | Adherent | Dulbecco's modified Eagle medium (DMEM)/F12-Ham's media, with 15% heat-inactivated fetal bovine serum, 1% non-essential amino acids (NEAA) and 1% L-glutamine (200 mM stock solution). | Evidence supporting a zoonotic origin of human coronavirus strain NL63 [97] |
| Rousettus aegyptiacus (Egyptian fruit bat). Fetus. Gender not specified | Head of the fetus Body of the fetus Vertebrate column of the fetus | Ro5T Ro6E Ro5R | Epithelial | Adherent | Dulbecco's modified Eagle medium (DMEM)/F12-Ham's media, with 5% gamma irradiated fetal calf serum and 1% L-glutamine (200 mM stock solution). | Cell lines from the Egyptian fruit bat are permissive for modified vaccinia Ankara [98] |
| Epomops buettikoferi (Büttikofer’s epauletted fruit bat) | Kidney | EpoNi/22.1 | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium (DMEM), with 10% heat inactivated fetal bovine serum, 1% L-glutamine (200 mM stock solution), 1% sodium pyruvate (100 mM stock solution) and 1% MEM non-essential amino acids (NEAA) (100 X stock solution). | Type I Interferon Reaction to Viral Infection in Interferon-Competent, Immortalized Cell Lines from the African Fruit Bat Eidolon helvum [99] |
| Eidolon helvum (African fruit bat). Adult. Gender not specified | Kidney | EidNi/41.3 | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium (DMEM), with 10% heat inactivated fetal bovine serum, 1% L-glutamine (200 mM stock solution), 1% sodium pyruvate (100 mM stock solution) and 1% MEM non-essential amino acids (NEAA) (100 X stock solution). | Type I Interferon Reaction to Viral Infection in Interferon-Competent, Immortalized Cell Lines from the African Fruit Bat Eidolon helvum [99] |
| Rousettus aegyptiacus (Egyptian fruit bat). Adult. Gender not specified | Kidney | RoNi7.1 | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium (DMEM), with 10% heat inactivated fetal bovine serum, 1% L-glutamine (200 mM stock solution), 1% sodium pyruvate (100 mM stock solution) and 1% MEM non-essential amino acids (NEAA) (100 X stock solution). | Type I Interferon Reaction to Viral Infection in Interferon-Competent, Immortalized Cell Lines from the African Fruit Bat Eidolon helvum [99] |
| Myotis daubentoniid (Vespertilionidae). Adult. Gender not specified | Lung | MyDauLu/47.1 | Epithelial | Adherent | Dulbecco's Modified Eagle's Medium (DMEM), with 10% heat inactivated fetal bovine serum, 1% L-glutamine (200 mM stock solution), 1% sodium pyruvate (100 mM stock solution) and 1% MEM non-essential amino acids (NEAA) (100 X stock solution). | Type I Interferon Reaction to Viral Infection in Interferon-Competent, Immortalized Cell Lines from the African Fruit Bat Eidolon helvum [99] |
Pregnant females can be identified by palpation and deeply anesthetized with isofluoran [98]. Following the surgical removal of the uteri, the animals are euthanized by bleeding. The diameter of the uteri is in the range of 2 cm. The explanted uteri must be placed immediately in phosphate-buffered saline (PBS) at ambient temperature and transferred to a tissue culture facility. The fetuses can be excised from the uteri within 6 hours post-surgical removal. Bat-derived cells can be aspirated under aseptic conditions with an 18-syringe needle gauge from the following tissues and/or organs: amniotic fluid, central nervous system-derived cells (brain and vertebral column), lung, kidney and liver. After aspiration, each cell suspension can be individually placed into tissue culture medium. The remaining body parts are minced, treated with recombinant cell-dissociation enzymes termed TrypLE (Gibco – Thermo Fisher Scientific, Grand Island, NY 14072, USA) and then placed into tissue culture medium, containing DMEM/F12 (Invitrogen), 5% (v/v) gamma-irradiated fetal calf serum (Biochrom – Merck, Kenilworth, NJ 07033) and L-glutamine 2mM final concentration (Gibco). For the first 5 passages, the tissue culture medium must be supplemented with recombinant human epidermal growth factor (hEGF) (Merck) at a final concentration of 20 ng/ml and LONG® R3 IGF-I human (Merck) at a final concentration of 10 ng/ml. Cell cultivation is carried out at 37 ◦C and 8% CO2.
Bat-derived cells can be immortalized with lentiviral vector-encoded simian virus 40 (SV40) large T antigen [99-103].
Bat-derived cell lines can be tested by reverse-transcription polymerase chain reaction (RT-PCR) for the presence of mycoplasma [99], filoviruses [104], lyssaviruses [105, 106], simian virus 5 (SV 5) [99] and coronaviruses [89, 90]. RT-PCR can also be utilized for the genotype of bat-derived cell lines by amplification of mitochondrial cytochrome b [99, 107, 108].
Also, induced induced pluripotent stem cells (iPSCs) have been generated from the wild greater horseshoe bats (Rhinolophus ferrumequinum) and the greater mouse-eared bats (Myotis myotis) [109]. The created iPSCs demonstrated similar features and had a gene expression profile similar to cells targeted by viruses. In addition, the obtained iPSCs had a high number of endogenous retroviral sequences. The study indicated that bats have developed tolerance mechanisms to resist a large load of viral sequences.
Bats have recently emerged as interesting animal models for the study of human diseases and ageing. A particular emphasis has been placed on the coronaviruses that have been responsible for the current Covid-19 pandemic and for the previous pandemics termed SARS and MERS. Studies can also be conducted for other viral agents, such as Ebola and Marburg viruses, Hendra virus and Nipah virus.
The biology of the bats is revealing distinct characteristics from other mammalian species, in terms of immune responses and slow progression of the ageing process. Studies have shown that immune responses and ageing process in bats seem to rely on different patterns of genetic and epigenetic expression that typically occur in all the other mammalian species [36].
Bats cannot be bred in the context of animal facilities. However, capturing bats require special precautions, in order to prevent the transmission of harmful infectious agents to the operators. Lastly, the feeding of the animals necessitates a particular dedication from the researchers, especially in the initial period, when the bats must be trained to feed from a Petri dish. On one hand, the lack of a syngeneic bat model can be perceived as a drawback. On the other hand, however, the type of studies on the immunology, slow progression of the ageing process and low incidence of cancer in bats can only be conducted on wild-type animals, albeit in captivity. Of course, bats must be carefully classified in the attempt to standardize as much as possible the results among different investigational settings. Disparities in the physiology of the bats is most probably going to affect the experimental outcomes. On these grounds, it is essential to use the same type of bats for all the experimental procedures to achieve consistent and reproducible findings.
- Marburg virus disease. WHO. Available from: www.who.int/health-topics/marburg-virus-disease/
- Ebola virus and Marburg virus. Mayo Clinic. Available from: www.mayoclinic.org/diseases-conditions/ebola-virus/symptoms-causes/syc-20356258
- Hendra virus infection. WHO. Available from: www.who.int/health-topics/hendra-virus-disease
- Nipah virus. WHO. Available from: www.who.int/news-room/fact-sheets/detail/nipah-virus
- Severe Acute Respiratory Syndrome (SARS). WHO. Available from: www.who.int/health-topics/severe-acute-respiratory-syndrome
- Coronavirus disease (COVID-19). WHO. Available from: www.who.int/emergencies/diseases/novel-coronavirus-2019
- Teeling E, Springer M, Madsen O, Bates P, O Brien S, Murphy W. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307:580-4 pubmed
- Morris S, Curtin A, Thompson M. Heterothermy, torpor, respiratory gas exchange, water balance and the effect of feeding in Gould's long-eared bat Nyctophilus gouldi. J Exp Biol. 1994;197:309-35 pubmed
- Jones G, Holderied M. Bat echolocation calls: adaptation and convergent evolution. Proc Biol Sci. 2007;274:905-12 pubmed
- Springer M, Teeling E, Madsen O, Stanhope M, de Jong W. Integrated fossil and molecular data reconstruct bat echolocation. Proc Natl Acad Sci U S A. 2001;98:6241-6 pubmed
- Wang Y, Pan Y, Parsons S, Walker M, Zhang S. Bats respond to polarity of a magnetic field. Proc Biol Sci. 2007;274:2901-5 pubmed
- Thomas S. Metabolism during flight in two species of bats, Phyllostomus hastatus and Pteropus gouldii. J Exp Biol. 1975;63:273-93 pubmed
- Austad S, Fischer K. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol. 1991;46:B47-53 pubmed
- Podlutsky A, Khritankov A, Ovodov N, Austad S. A new field record for bat longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1366-8 pubmed
- Ronald M. Nowak. Walker's Mammals of the World. Available from: jhupbooks.press.jhu.edu/title/walkers-mammals-world
- Wilkinson G, South J. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124-31 pubmed
- Huang Z, Jebb D, Teeling E. Blood miRNomes and transcriptomes reveal novel longevity mechanisms in the long-lived bat, Myotis myotis. BMC Genomics. 2016;17:906 pubmed
- Drews R. [Prof. Tadeusz Jacyna-Onyszkiewicz (1906-1972)]. Pol Przegl Chir. 1974;46:955-6 pubmed
- Estensen R, Baserga R. The influence of the age of the host on the incidence of blood-borne metastases. Cancer Res. 1961;21:799-802 pubmed
- Romano G, Prisco M, Zanocco Marani T, Peruzzi F, Valentinis B, Baserga R. Dissociation between resistance to apoptosis and the transformed phenotype in IGF-I receptor signaling. J Cell Biochem. 1999;72:294-310 pubmed
- Romano G. The complex biology of the receptor for the insulin-like growth factor-1. Drug News Perspect. 2003;16:525-31 pubmed
- Brian D, Baric R. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1-30 pubmed
- Dudouet B, Robine S, Huet C, Sahuquillo Merino C, Blair L, Coudrier E, et al. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol. 1987;105:359-69 pubmed
- Ontiveros E, Kim T, Gallagher T, Perlman S. Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein. J Virol. 2003;77:10260-9 pubmed
- Li F, Li W, Farzan M, Harrison S. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864-8 pubmed
- Yeager C, Ashmun R, Williams R, Cardellichio C, Shapiro L, Look A, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420-2 pubmed
- Mbala Kingebeni P, Villabona Arenas C, Vidal N, Likofata J, Nsio Mbeta J, Makiala Mandanda S, et al. Rapid Confirmation of the Zaire Ebola Virus in the Outbreak of the Equateur Province in the Democratic Republic of Congo: Implications for Public Health Interventions. Clin Infect Dis. 2019;68:330-333 pubmed publisher
- Asad A, Aamir A, Qureshi N, Bhimani S, Jatoi N, Batra S, et al. Past and current advances in Marburg virus disease: a review. Infez Med. 2020;28:332-345 pubmed
- Gonzalez J, Pourrut X, Leroy E. Ebolavirus and other filoviruses. Curr Top Microbiol Immunol. 2007;315:363-87 pubmed
- To capture a bat. CDC. Available from: www.cdc.gov/rabies/bats/contact/capture.html
- CDC: Bats pose biggest rabies threat in US. The University of Minnesota. Available from: www.cidrap.umn.edu/news-perspective/2019/06/cdc-bats-pose-biggest-rabies-threat-us
- Canadian Council on Animal Care: Guide to the Care and Use of Experimental Animals. Available from: ccac.ca/Documents/Standards/Guidelines/Vol2/bats.pdf
- Constantine D. Geographic translocation of bats: known and potential problems. Emerg Infect Dis. 2003;9:17-21 pubmed
- Racey P. The breeding, care and management of vespertilionid bats in the laboratory. Lab Anim. 1970;4:171-83 pubmed
- Approved euthanasia methods for bats. USGS. Available from: www.usgs.gov/media/videos/approved-euthanasia-methods-bats-microchiroptera-audio-described
- Mourya D, Lakra R, Yadav P, Tyagi P, Raut C, Shete A, et al. Establishment of cell line from embryonic tissue of Pipistrellus ceylonicus bat species from India & its susceptibility to different viruses. Indian J Med Res. 2013;138:224-31 pubmed
- Tonini T, Claudio P, Giordano A, Romano G. Transient production of retroviral- and lentiviral-based vectors for the transduction of Mammalian cells. Methods Mol Biol. 2004;285:141-8 pubmed
- Tonini T, Claudio P, Giordano A, Romano G. Retroviral and lentiviral vector titration by the analysis of the activity of viral reverse transcriptase. Methods Mol Biol. 2004;285:155-7 pubmed
- Tonini T, Claudio P, Giordano A, Romano G. Determination of functional viral titer by drug-resistance colony assay, expression of green fluorescent protein, and beta-galactoside staining. Methods Mol Biol. 2004;285:149-53 pubmed
- Hofmann A, Kessler B, Ewerling S, Kabermann A, Brem G, Wolf E, et al. Epigenetic regulation of lentiviral transgene vectors in a large animal model. Mol Ther. 2006;13:59-66 pubmed
- Panning M, Laue T, Olschlager S, Eickmann M, Becker S, Raith S, et al. Diagnostic reverse-transcription polymerase chain reaction kit for filoviruses based on the strain collections of all European biosafety level 4 laboratories. J Infect Dis. 2007;196 Suppl 2:S199-204 pubmed
- Heaton P, Johnstone P, McElhinney L, Cowley R, O Sullivan E, Whitby J. Heminested PCR assay for detection of six genotypes of rabies and rabies-related viruses. J Clin Microbiol. 1997;35:2762-6 pubmed
- Irwin D, Kocher T, Wilson A. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991;32:128-44 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
