This is a knockout-validated antibody summary, based on the three publications "The steroid receptor coactivator-3 is required for developing neuroendocrine tumor in the mouse prostate" [1], "Tead and AP1 Coordinate Transcription and Motility" [2], and "Knockout of SRC-1 and SRC-3 in Mice Decreases Cardiomyocyte Proliferation and Causes a Noncompaction Cardiomyopathy Phenotype" [3]. Labome curates formal publications to compile a list of antibodies with unambiguous specificity within Validated Antibody Database (VAD).
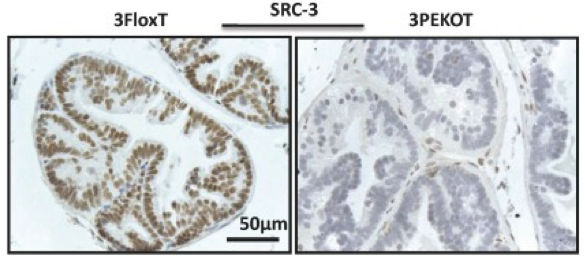
Antibody: SRC-3
Catalog number: 2126
Summary: Rabbit monoclonal raised against a synthetic peptide corresponding to the sequence of human SRC-3 protein. Detects endogenous levels of total SRC-3 protein (all isoforms) from human, mouse, rat, and monkey. Used for western blotting, immunoprecipitation, and chromatin IP.
Company: Cell Signaling
IHC, immunohistochemistry
Fixed prostate tissues sections (paraffin embeded) prepared from 8-week-old floxed (3FloxT) and specifically ablated in AR+/Syp- prostatic epithelial cells (3PEKOT) mice.
Biotinylated anti-Rabbit IgG (Vector labs) used at 1:400 dilution
Signal was enhanced using the VECTASTAIN ABC system and visualized with DAB kit.
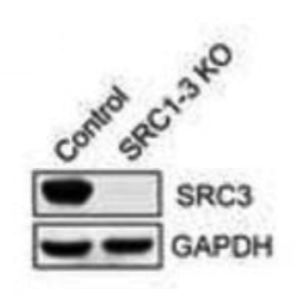
Western blot
Control and SRC1-3 KO HCT116 cells.
HRP conjugated secondary antibodies (Jackson Laboratories).
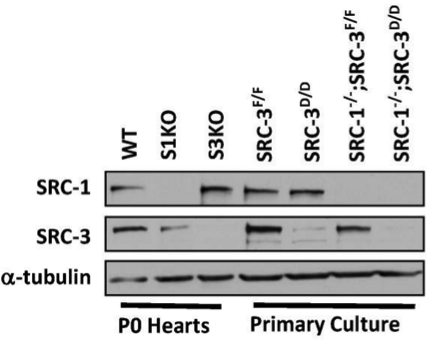
Western blot
WT, SRC-1 knockout and SRC-3 knockout hearts at P0, SRC-3f/f, SRC-3d/d, S1KO;SRC-3f/f and S1KO;SRC-3d/d primary cardiomyocytes. Frozen tissue powders and harvested cells were extracted with RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 0.25% Na-deoxycholate.
Appropriate secondary antibodies conjugated with horseradish peroxidase.
ECL reagent kit.
If the antibody described in this summary is a polyclonal antibody, since polyclonal antibodies are of limited quantity, please inquire the supplier whether any current polyclonal antibody with the same catalog number is exactly the same as the one described in this summary. Sometimes, different bleeds or different animals are used, usually with a different lot number. In such cases, the result in this summary may not apply to the new antibody with the same catalog number.
- product
