product summary
Loading...
company name :
Tocris Bioscience
product type :
chemical
product name :
HC 067047
catalog :
4100
quantity :
10 mg (also 50 mg)
price :
223 USD
more info or order :
citations: 13
| Reference |
|---|
image
image 1 :
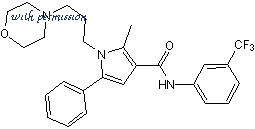
Cat.No. 4100 - HC 067047 C26H28F3N3O2 CAS No. 883031-03-6
product information
brand :
Tocris
master code :
4100
SKU :
4100/10
product name :
HC 067047
description :
Potent and selective TRPV4 antagonist
target :
TRPV Antagonists
category :
Small Molecules
unit size :
10 mg (also 50 mg)
purity :
99%
observed molecular weight :
471.51
url print :
?utm_source=distributor&utm_medium=referral&utm_campaign=product&utm_term=smallmolecules
details of functionality :
Potent and selective TRPV4 antagonist. Reversibly inhibits currents through mouse, human and rat TRPV4 orthologs (IC50 values are 17, 48 and 133 nM respectively). Also inhibits the endogenous TRPV4-mediated response to 4 -PDH (IC50 = 22 nM). Selective for TRPV4 over TRPV1, TRPV2, TRPV3 and TRPM8 channels.
catalog number base :
4100
product keywords :
HC067047 trpv4 transient receptor potential channels potent selective antagonists TRPV 4100,
extended description :
Potent and selective TRPV4 antagonist
chemical name text :
2-Methyl-1-[3-(4-morpholinyl)propyl]-5-phenyl-N-[3-(trifluoromethyl)phenyl]-1H-pyrrole-3-carboxamide
formula :
C 26 H 28 F 3 N 3 O 2
formula text :
C26H28F3N3O2
cas num :
883031-03-6
2020 USD :
214
2021 USD :
223 USD
storage :
Store at +4°C
more info or order :
company information

Tocris Bioscience
The Watkins Building
Atlantic Road
Avonmouth, Bristol
BS11 9QD
Atlantic Road
Avonmouth, Bristol
BS11 9QD
info@bio-techne.com
https://www.tocris.com800-343-7475
headquarters: UK
browse more products
questions and comments
