product summary
Loading...
company name :
Tocris Bioscience
product type :
chemical
product name :
1231/5
catalog :
1231/5
quantity :
5 mg
price :
195 USD
more info or order :
citations: 13
| Reference |
|---|
image
image 1 :
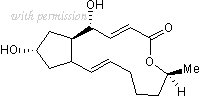
Cat.No. 1231 - Brefeldin A C16H24O4 CAS No. 20350-15-6
product information
master code :
1231
SKU :
1231/5
product name :
Brefeldin A
unit size :
5 mg
description :
Disrupts protein translocation to Golgi
target :
Translocation, Exo/Endocytosis Products
category :
Small Molecules
purity :
98%
observed molecular weight :
280.36
url print :
?utm_source=labome&utm_medium=referral&utm_campaign=product&utm_term=smallmolecules
details of functionality :
Brefeldin A is a reversible inhibitor of protein translocation from the endoplasmic reticulum (ER) to the Golgi apparatus. Blocks binding of ADP-ribosylation factor (ARF1) to the Golgi apparatus and inhibits GDP-GTP exchange, leading to activation of ER stress signaling pathways. Can be used to induce autophagy in mammalian cells. Also enhances CRISPR-mediated homology-directed repair (HDR) efficiency ~2-fold when applied at 100 nM, in human induced pluripotent stem cells (iPSCs). Antifungal.
top caption :
Disrupts protein translocation to Golgi
extended description :
Disrupts protein translocation to Golgi
chemical name text :
1,6,7,8,9,11a ,12,13,14,14a -Decahydro-1 ,13 -dihydroxy-6 -methyl-4H-cyclopent[f]oxacyclotridecin-4-one
formula :
C16H24O4
formula text :
C16H24O4
cas num :
20350-15-6
USD :
195 USD
product details :
Disrupts protein translocation to Golgi
storage :
Store at -20░C
more info or order :
company information

Tocris Bioscience
The Watkins Building
Atlantic Road
Avonmouth, Bristol
BS11 9QD
Atlantic Road
Avonmouth, Bristol
BS11 9QD
info@bio-techne.com
https://www.tocris.com800-343-7475
headquarters: UK
browse more products
questions and comments
