product summary
Loading...
company name :
Tocris Bioscience
product type :
chemical
product name :
Fluvoxamine maleate
catalog :
1033/10
quantity :
10 mg (also 50 mg)
price :
90 USD
more info or order :
citations: 9
| Reference |
|---|
Su D, Jiang R, Liu N, Ding L, Wang D, Yu H, et al. Effects of BD1047, a ?1 receptor antagonist, on the expression of mTOR, Camk2? and GSK-3? in fluvoxamine-treated N2a cells. Exp Ther Med. 2014;7:435-438 pubmed
|
Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, et al. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695-702 pubmed
|
Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by various antidepressant drugs. Neuropsychopharmacology. 2004;29:1841-51 pubmed
|
image
image 1 :
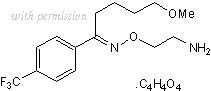
Cat.No. 1033 - Fluvoxamine maleate C15H21F3N2O2.C4H4O4 CAS No. 61718-82-9
product information
brand :
Tocris
catalog number base :
1033
SKU :
1033/10
product name :
Fluvoxamine maleate
units size :
10 mg (also 50 mg)
description :
5-HT reuptake inhibitor
target :
5-HT Transporter Inhibitors
category :
Small Molecules
purity :
99%
observed molecular weight :
434.41
url print :
?utm_source=biocompare&utm_medium=referral&utm_campaign=product&utm_term=smallmolecules
details of functionality :
Fluvoxamine maleate is a selective serotonin reuptake inhibitor; antidepressant. Binds to the human 5-HT transporter with a Ki of 1.6 nmol/l.
top caption :
5-HT reuptake inhibitor
extended description :
5-HT reuptake inhibitor
chemical name text :
(E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanone-O-(2-aminoethyl)oxime maleate
formula :
C15H21F3N2O2.C4H4O4
formula text :
C15H21F3N2O2.C4H4O4
cas num :
61718-82-9
USD :
90 USD
product details :
5-HT reuptake inhibitor
storage :
Desiccate at +4°C
more info or order :
company information

Tocris Bioscience
The Watkins Building
Atlantic Road
Avonmouth, Bristol
BS11 9QD
Atlantic Road
Avonmouth, Bristol
BS11 9QD
info@bio-techne.com
https://www.tocris.com800-343-7475
headquarters: UK
browse more products
questions and comments
