product summary
Loading...
company name :
Tocris Bioscience
product type :
chemical
product name :
0877/10
catalog :
0877/10
quantity :
10 mg (also 50 mg)
price :
219 USD
more info or order :
citations: 17
| Reference |
|---|
Silva C, Tamura E, Macedo S, Cecon E, Bueno Alves L, Farsky S, et al. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br J Pharmacol. 2007;151:195-205 pubmed
|
Savinainen J, Kokkola T, Salo O, Poso A, Jarvinen T, Laitinen J. Identification of WIN55212-3 as a competitive neutral antagonist of the human cannabinoid CB2 receptor. Br J Pharmacol. 2005;145:636-45 pubmed
|
Roy D, Angelini N, Fujieda H, Brown G, Belsham D. Cyclical regulation of GnRH gene expression in GT1-7 GnRH-secreting neurons by melatonin. Endocrinology. 2001;142:4711-20 pubmed
|
Sjöblom M, Jedstedt G, Flemstrom G. Peripheral melatonin mediates neural stimulation of duodenal mucosal bicarbonate secretion. J Clin Invest. 2001;108:625-33 pubmed
|
image
image 1 :
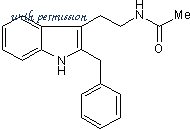
Cat.No. 0877 - Luzindole C19H20N2O CAS No. 117946-91-5
product information
master code :
877
SKU :
0877/10
product name :
Luzindole
unit size :
10 mg (also 50 mg)
description :
Competitive melatonin MT1/MT2 antagonist
target :
Melatonin (MT) Receptor Antagonists
category :
Small Molecules
purity :
98%
observed molecular weight :
292.38
url print :
?utm_source=labome&utm_medium=referral&utm_campaign=product&utm_term=smallmolecules
details of functionality :
Luzindole is a melatonin antagonist.
top caption :
Competitive melatonin MT1/MT2 antagonist
extended description :
Competitive melatonin MT1/MT2 antagonist
chemical name text :
N-Acetyl-2-benzyltryptamine
formula :
C19H20N2O
formula text :
C19H20N2O
cas num :
117946-91-5
USD :
219 USD
product details :
Competitive melatonin MT1/MT2 antagonist
storage :
Store at -20░C
more info or order :
company information

Tocris Bioscience
The Watkins Building
Atlantic Road
Avonmouth, Bristol
BS11 9QD
Atlantic Road
Avonmouth, Bristol
BS11 9QD
info@bio-techne.com
https://www.tocris.com800-343-7475
headquarters: UK
related products
browse more products
questions and comments
