product summary
Loading...
company name :
Sino Biological
product type :
protein
product name :
Influenza A H1N1 (A/California/07/2009) Hemagglutinin / HA Protein (His Tag)
catalog :
11085-V08H
quantity :
100 µg
price :
348 USD
more info or order :
citations: 17
| Published Application/Species/Sample/Dilution | Reference |
|---|---|
| |
image
image 1 :
product information
Catalog Number :
11085-V08H
Product Name :
Influenza A H1N1 (A/California/07/2009) Hemagglutinin / HA Protein (His Tag)
Product Type :
Protein
Host Species :
HEK293 Cells
Conjugation :
C-His
Size :
100 µg
List Price :
348 USD
Gene ID :
ACP41953.1
Product Description :
A DNA sequence encoding the extracellular domain of Influenza A virus H1N1 (A/California/07/2009) (ACP41953.1) hemagglutinin (Met1-Gln529) (HA1+HA2, uncleaved) was fused with a polyhistidine tag at the C-terminus.
SpeciesSummary :
H1N1
ALTnames :
HA,Hemagglutinin/HA
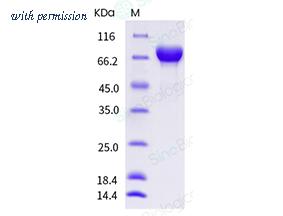
Purity :
> 95 % as determined by SDS-PAGE.
Buffer :
Lyophilized from sterile PBS, pH 7.4.
Packaging :
In general, recombinant proteins are provided as lyophilized powder which are shipped at ambient temperature. Bulk packages of recombinant proteins are provided as frozen liquid. They are shipped out with blue ice unless customers require otherwise.
Storage :
Samples are stable for up to twelve months from date of receipt at -20℃ to -80℃. Store it under sterile conditions at -20℃ to -80℃. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
more info or order :
company information

Sino Biological
Building 9, No.18 Kechuang 10th St, BDA, Beijing, 100176
marketing@sinobiological.com
https://www.sinobiological.com86 400-890-9989
headquarters: China
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
related products
browse more products
- Influenza A H2N2 (A/Japan/305/1957) Hemagglutinin / HA Protein (His Tag)
- Human / Mouse / Rat / Rhesus / Canine BMP-2 / BMP2A Protein | 10426-HNAE
- Human HER2 / ErbB2 Protein (His Tag) | 10004-H08H
- Human PDGFRa / CD140a Protein (His Tag) | 10556-H08H
- Human Carboxypeptidase E / CPE Protein (His Tag) | 10069-H08H
questions and comments
