A comprehensive review of the adenoviral vector system for protein expression. Please note in both gene therapies and laboratory experiments, the adenoviral vector system is being replaced by vector systems based on adeno-associated viruses in recent years.
A large number of acute respiratory, gastrointestinal and eye infections in humans are caused by adenoviruses [3, 4]. Adenoviruses have a wide host range from monkeys, mice to humans. Adenoviruses are powerful research tools for investigating virological and cellular events and also often carriers for new vaccines, such as vaccines against SARS-CoV 2 [5]. Adenoviruses have 50 different serotypes; however majority of molecular information about host-cell interaction is based on studies related to 2 and 5 [6-8]. Hence, the most commonly used adenoviral vectors are derived from human adenovirus serotypes 2 and 5 for in vitro and in vivo gene delivery [9]. Different serotypes may work well with different tissues and/or delivery route [10]. For example, Wienert B et al used a replication-deficient adenoviral-serotype 5 (dE1/E3) backbone to edit genes in mice [11]. Hulin-Curtis SL et al applied the same backbone to introduce oligopeptides for oncolytic virotherapies [12].
Table I compares various recombinant viral vectors used for gene delivery. There has been a rapid expansion in the use of adenoviral vectors for in vitro and in vivo gene delivery [13]. It is used for 1) gene therapy [14, 15] ; 2) molecular tool to study gene expression, both in vitro and in vivo expression in difficult-to-transduce cell types and tissues [16], for example, retrograde introduction of optogenetic channelrhodopsin 2 [17] or introduction of targeted mutagenesis in combination with gene-editing tools such as CRISPR [18] ; 3) the production of high levels of recombinant, potentially therapeutic proteins; and 4) in vivo vaccination [19], for example, chimpanzee adenovirus vector Ebola vaccine [20], flu vaccine [21], or MERS-CoV vaccine [22]. However, the usage of adenoviral systems are being replaced by adeno-associated viral systems except in the case of vaccine deveopment.
| Adenoviruses | Retroviruses | Lentiviruses | |
|---|---|---|---|
| Cell-specificity | Dividing and non-dividing | Dividing | Dividing and non-dividing |
| Stability | Epichrosomosal, not replicated with cell division | Integrates into host genome Limitations: Integration into the host genome is a random unpredictable event, hence depending on the site of insertion, cellular function may be disturbed due to genetic disruption, concerns for insertional mutagenesis causing activation of oncogenes have also been observed | Integrates into host genome |
| Transgene expression | Transient Limitations: Best suited for in vitro use including primary cells, quick testing of a target cells, quick testing of a target before committing to transgenic mouse model | Stable | Transient/Stable |
| Immune response | High Remarks: Gutless vectors are being redesigned to reduce immunogenicity | Moderate | Low |
| Viral titer | High Remarks: Suitable for both in vitro including primary cells and in vivo use | Low | Moderate Remarks: Best for in vivo use, in vitro use not advised because high titers similar to in vivo use are required for in vitro transduction as well |
| Relative ease of construction | Step by step rescue using two-plasmid system and co-transfection in packaging cell lines | Step wise cloning to rescue transgene and co-transfection in packaging cell lines Comments: Depends on replication-competent or replication-defective vectors | Multiple plasmids encoding required proteins are co-transfected into packaging cell line Limitations: The final vector stock cannot be reused to amplify due to hazard of generating replication competent virus, hence everytime generation of vector is initiated with co-transfection step |
| Efficiency of transduction | High and wide range of cell types | Low | Moderate |
However, host immune response to viral proteins has been a recurring issue raising concerns about adenovirus-mediated gene delivery particularly in the context of gene therapy. Therefore, research efforts to understand the adenovirus structure and host cell interactions are ongoing to redesign viral vectors to circumvent these significant problems [23-25].
All adenoviruses are comprised of a naked icosahedral protein shell (70-100nm) in diameter, encapsidating a 36kb linear, double-stranded DNA with inverted terminal repeat (ITR) sequences at each end. The exact shape and size of the virion and genome can vary greatly among adenoviruses with different host specificity. The virus life cycle initiates with attachment to cell surface receptors. (I) Attachment: The virus attaches to the cell surface receptors via interaction of the pentons with alphaVbeta3 and alphaVbeta5 integrin proteins. (II) Internalization: It is subsequently internalized by receptor-mediated endocytosis, escapes from endosome to the cytosol and translocates to the nucleus to begin viral transcription and replication. (III) Replication: Cell death is triggered to release viral progeny upon completion of the viral life cycle. Transcriptional units are conventionally referred to as early (E1a, E1b, E2, E3, and E4), delayed early (proteins IX and IVa2) or late genes (L1-L5) based on the temporal expression relative to the onset of viral DNA replication. The early gene products are generally involved in gene transcription, replication, host immune suppression and apoptosis inhibition of host cell, and late gene products are responsible for virion assembly.
Most recombinant manipulations of adenoviruses are limited to regions not essential for viral production such as E1, E2A, E3, and E4. Earlier methods involved direct manipulation of viral DNA extracted from virions. In general, these methods involved in vivo homologous recombination between viral DNAs cotransfected into cells [26] and in vitro ligation of viral DNAs cleaved by restriction enzymes [27, 28]. However, a major limitation of these methods was the introduction of precise alterations into the large adenoviral genome restricting efficient cloning manipulation in the viral DNA. Next generation viral vectors were based on the two-plasmid rescue system [29]. Vectors in this method were generated by homologous recombination in vivo between two noninfectious plasmids cotransfected into 293 cells. It has been widely used due to its simplicity. The major limitation of this method is the low frequency of the recombination event and the potential instability of the large plasmid due to the presence of head-to-head ITR repeats. Another method based on the manipulation of the entire viral genome in mammalian systems is via yeast artificial chromosome (YAC) [30]. Homologous recombination between YAC and infective virions is used to generate a targeted modification in the viral genome. However, the shortcomings of this approach are the use of an additional host, yeast, and low DNA yield.
Manipulation of large adenoviral genome is limited by the use of classical molecular biology techniques. Homologous recombination provides an alternate approach and flexibility to manipulate large DNA. This method requires a 'shuttle' and a 'backbone' plasmid. A typical shuttle vector usually contains the 5’ end of the adenoviral genome wherein the E1, and other non-essential viral genes are replaced by a gene of interest (GOI) or transgene using conventional cloning techniques. This shuttle vector subsequently is recombined with 'backbone vector'. The backbone vector is the primary carrier of the majority of the viral genome but importantly lacks essential genes for viral propagation in naturally occurring cells. The recombination event results in a single DNA molecule encoding all genes required for viral replication and production exclusively in 'packaging cell lines' and not in naturally occurring cells. This recombination process was accomplished through homologous recombination in mammalian cells traditionally which proved to be rate-limiting owing to the inefficient and somewhat unpredictable nature of homologous recombination in mammalian cells [31]. Hence, systems such as yeast and microbial models for recombination have been explored. Once DNA incorporating both backbone and shuttle vector sequences containing GOI have been generated in microbial systems, the entire cloned DNA is transfected into mammalian packaging cell lines whose function is to complement E1 gene essential for viral replication. These next generation and modified methods have made it possible to generate large quantities of recombinant adenoviruses in a timely and predictable manner.
Homologous recombination, direct ligation and, cosmid technology are three basic approaches that enable large viral genome manipulation as a stable plasmid and efficient construction of precisely targeted and infectious adenovirus in E. coli [32-36]. Advantages of these techniques are:
- Viral genome can be manipulated at any point.
- Individual bacterial clones are used for purification of recombinant viral DNA which ensures generation of homogenous virus preparations.
- These methods separate viral vector construction from production.
The first step of this approach is performed in a bacterial system and the second step takes place in a mammalian complementation cell line. Its advantage is the flexibility to control and optimize each step.
In 2007, Adeasy system was described by Luo and colleagues [37] in which they modified the protocol based on high efficiency of homologous recombination in a specific bacterial strain along with selective antibiotic resistance marker to simplify recombinant vector production. Table II summarizes commercial systems that are based on the above principles and comparison of technology used in addition to description of shuttle and vectors used in each system. The major focus on the development of these systems is to streamline the generation time of the virus along with efforts to reduce RCA generation during the scale-up. To give a basic idea about virus generation inclusive of all cloning and recombination events, AdEasy system is described below. All systems have incorporated slight modifications of methodology either at the subcloning or homologous recombination systems, yet the basic principle remains the same.
| AdEasy | Adeno-X | Virapower (pAD/CMV/DEST) | RAPAd-CMV | |
|---|---|---|---|---|
| Company | Qbiogene/Stratagene/Agilent | Clontech / Takara Bio | Invitrogen | Cell Biolabs Inc. |
| Principle | Homologous recombination with AdV backbone in bacteria | In vitro ligation with AdV backbone | Direct cloning of GOI into AdV backbone | Homologous recombination with AdV backbone in 293 cells |
| Vector shuttle | pShuttle-CMV-lacZ, pShuttle, pShuttle-CMV Modifications:Monitor expression at single cell level, pshuttle-IRES-hrGFP1, pshuttle-IRES-hrGFP2 | pCMV-Shuttle2 Modifications:Available in constitutive and tet-inducible formats | No shuttle Pros: Direct ligation into backbone | pacAd5-Shuttle2, LacZ and GFP reporters available |
| Vector backbone | pAdEasy Modifications:Replication incompetent ΔE1/ΔE3 Ad5 to lower RCA | Adeno-X Modifications:With or without GFP reporters, Replication incompetent ΔE1/ΔE3 Ad5 to lower RCA | pAD/DEST, pAD/CMV/V5-DEST, pAD/CMV/V5-GW/lacZ | pacAd5 Modifications:Lacks left hand ITR, packaging signal and E1 sequences to lower RCA |
| Technology |
|
|
|
|
| Pros | Pre-transformed with pAdEasy to reduce background by shuttle vector, high transduction efficiency, high recombinants, streamline time constraints, reduce RCA | In vitro ligation reducing one step compared to pAdEasy system, Adeno-X adenoviral system 3 uses expression cassette to directly clone onto pAd backbone eliminating subcloning in shuttle vector | Fast, efficient, plasmid contains recombination sites for cloning GOI, eliminates subcloning by direct insertion of GOI into pAd backbone, Adapted gateway recombinant cloning tech | Modified homologous recombination in mammalian cells, unlike traditional methods no plaque screening, eliminates in vitro recombination in bacterial cells |
| Cons | High fidelity, screening of potential recombinants, Several steps | Screening of potential recombinants, Several steps | Modified to minimize RCA, Recombination is in 293 cells without plaque purification | |
| Additional formats | AdEasy XL Adenoviral system | Adeno-X Expression System 1 Knockout Adenoviral RNAi System 1 Adeno-X Adenoviral System 3 (CMV) | Virapower (pAD/CMV/DEST) | RAPAd CMV Adenoviral Expression Systems miRNA expression systems |
| Reference | [38-41] | [42] | [43-45] | [46] |
The Adeasy system [37] has three steps. 1) Cloning GOI into the shuttle vector: The shuttle vectors are commercially available. Most common shuttle vector is pshuttle-CMV. 2) Homologous recombination in vivo in bacteria: Linearize shuttle vector containing GOI and introduce into highly electrocompetent BJ5183 bacterial cells (Stratagene, cat no BJ5183-AD-I) pretransformed with supercoiled backbone plasmid vector containing most of the viral genome flanked by inverted terminal repeat (ITR) to facilitate homologous recombination. 3) Virus production in packaging cell line: Transfection of PacI-digested linearized recombinant adenoviral DNA containing GOI into packaging cell line, HEK-293 cells, and harvest viruses 14-20 days later.
Primarily four different modifications of the basic shuttle vector can be used by any researcher. They contain different resistance cassettes: ampicillin and kanamycin. The basic vector is pShuttle multiple cloning site (MCS) with the highest capacity to accommodate a transgene (up to 7.5 kb) coupled with the flexibility to customize promoter and termination signals to drive transgene expression. The pshuttle-CMV vector contains a multiple cloning site (MCS) suitable for insertion of large cDNA (6.6 kb) sandwiched between CMV promoter and SV40 polyadenylation signal. Besides, the vector contains stretches of sequence homologous to pAdEasy-1 to facilitate homologous recombination. Short inverted terminal repeats, R-ITR and L-ITR are present which have roles in viral DNA replication.
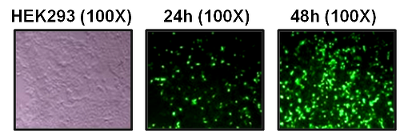
Another modification on pshuttle and pshuttle CMV is a GFP cassette to enable easy screen of transgene expression, for example, pshuttle-IRES-hrGFP-1 and pshuttle-IRES-hrGFP-2. Both vectors contain the CMV promoter and a bicistronic expression cassette in which the MCS is followed by ECMV-IRES, directing translation of recombinant fluorescent protein (hrGFP) as a second open reading frame. This enables expression of GOI (up to 5.2 kb) to be monitored at the single-cell level owing to the hrGFP expression on the same transcript. However, the introduction of the GFP cassette diminishes the capacity for larger GOI. Figure 1 shows an example of transgene expression monitoring by visualizing GFP expression in HEK293 cells used from Zhao et al , 2010 [1].

pAdEasy-1: Most commonly used adenoviral backbone vector, pAdEasy-1 retains a majority of the human adenovirus serotype 5 (Ad5) genome and has double-deletion for EI and E3 genes which creates space for the transgene and eliminates self-replication capacity. This enables propagation of AdEasy-1 derived recombinant adenoviruses in packaging cell lines, HEK-293 cells or AD-293 cells, which contain the E1 gene necessary for viral replication. The E3 region encodes for proteins involved in evading host immunity and is dispensable. It also carries ampicillin resistance which is lost after recombination with shuttle vector.
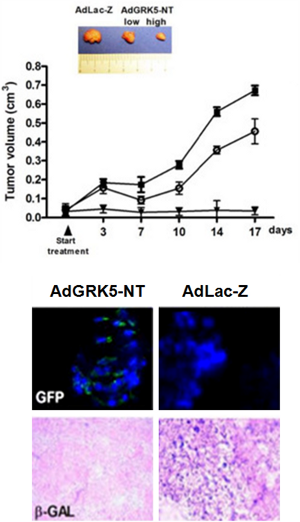
pAdEasy-2: Less commonly used, pAdEasy-2 lacks E1, E3 and E4 [35]. Propagation of AdEasy-2 derived recombinant adenoviruses require packaging cell lines containing both E1 and E4. However, this vector has downsides, of which most important is the difficulty in generating high-titer AdEasy-2 derived viruses. The lacZ gene is inserted into the MCS of the pShuttle-CMV to produce pShuttle CMV. This construct is primarily used as a control for the production of recombinant adenovirus. Figure 2 shows an example of using Ad-LacZ as control adenoviral vector in vivo to assess relative transgene expression in the same tissue in an in vivo model [2].
Gutless backbone vectors: These vectors are used for expressing a large transgene or multiple transgenes [47]. Gutless vectors can tolerate much larger replacements in the adenoviral backbone, unlike AdEasy vector systems which can tolerate replacements only in E1 and E4 region. These vectors contained only the ITRs and packaging sequence of the viral genome, and the entire sequence is replaced by exogenous sequences resulting in so-called gutted or gutless vectors; gene products for viral replication and packaging are provided in trans. Gutless vectors can accommodate up to 35 kb foreign DNA, display significantly less host immune response and attain long-term expression of multiple transgenes in a single vector.
Figure 3 is a schematic illustration describing primary events required to generate recombinant adenoviral vectors.
Cloning DNA in a shuttle vector should follow standard molecular biology practices: for example, identify a suitable restriction site on the MCS and gene of interest; design primers that contain restriction sites at the end so that sticky ends can be created to facilitate directional cloning; PCR-amplified target sequence must be validated by automated DNA sequencing. If blunt end ligation performed between target and shuttle vector, the presence of transgene must be confirmed by restriction digest/PCR amplification/DNA sequencing. Validation by all three methods is the best approach. In some cases, investigators also perform functional assays by transfecting cells and confirm by western blot or activity assays if the GOI has any testable biochemical function. It is advisable to at least perform a western blot expression analysis of the transgene at this stage because many unanticipated problems at later stages may be contributed due to problems of GOI cloning and expression at this first step. Depending upon the shuttle vector of choice, for example, for cloning GOI in pShuttle vector, a promoter and SV40 polyadenylation signal must be incorporated in the transgene cassette to drive and terminate transcription. For all shuttle vectors, a consensus Kozak signal sequence is important to include for efficient transgene expression.
This step can be performed in two ways depending on the type of BJ5183 cells used. BJ5183 are highly electrocompetent bacterial cells which can be co-transformed with pAdEasy-1 and the linearized pShuttle vector containing GOI with Pme I. BJ5183-AD-1, which already harbors the pAdEasy-1 backbone, can be used for a linearized pShuttle vector containing GOI.

Comment: The use of high-quality electrocompetent cells is highly recommended. BJ5183 cells exhibit low transformation efficiency and high frequency of homologous recombination than most conventional strains used for molecular cloning. This step requires the use of an electroporator or equivalent apparatus. After transformation, follow basic molecular methods and grow the bacteria on a plate. It is important to pick the smallest, well-isolated colonies and grow them in starter culture containing kanamycin. Perform mini-preps. Isolate DNA and perform restriction digest with PacI to confirm the presence of desired recombinants. Correct recombinants yield a smaller fragment, 3.0-4.5 kb and a larger fragment of approximately 30 kb. The yield of recombinant DNA is much lower than the noise which generally results from unwanted recombination events. Figure 4 shows a schematic depiction of agarose gel and restriction profile of potential adenoviral recombinants.

After the validation of desired recombinant clones, DNA is used to retransform DH10B cells. Grow mini-prep and extract DNA. Validate desired recombinant DNA by restriction digest. Once confirmed, the final step is to grow maxi-prep and use a commercially available kit to extract recombinant adenoviral DNA. It is advisable to perform restriction digest and also PCR amplification to validate and confirm the integrity of the transgene.
Low passage number HEK-293 cells should be grown up to 50-70% confluency at the time of transfection with the recombinant adenoviral DNA. Digest recombinant adenoviral plasmid DNA with PacI and use lipofectamine for transfection following manufacturer’s recommendations. Harvest viruses between 14-20 days post-transfection depending upon viral plaques or cytopathic effect (CPE) which is visible by light microscopy. Scrape cells off from flasks using a cell scraper and transfer to a conical tube to prepare cell lysates. Collect cells after a short spin at a low speed and resuspend in a small volume to release adenovirus from cells after freeze-thaw cycles. Centrifuge and pellet cell debris. Store virus cell lysates at -80°C if not immediately used.
Comments: A dedicated incubator should be used for adenovirus-treated cells to separate infected cells from non-infected ones. Any tools, apparatus and tabletop surface including BSL2 hoods coming in contact with the virus should be disinfected and decontaminated. Similarly, waste generated should be disinfected and decontaminated before disposal.
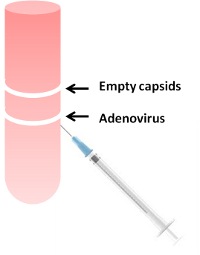
Figure 5 depicts an overview of the amplification phase. For use in cell culture or primary cells, highly purified virus is not necessary; viral titer must be determined to augment correct dosing for different experimental conditions. Figure 6 depicts a cartoon of separation of recombinant adenoviruses in contrast to empty capsids and defective viruses on a CsCl gradient. For a more detailed protocol, please refer to the links provided at the end for manufacturer’s recommendations.
Tail vein injections are traditional routes for intravenous delivery, although it is technically challenging. Besides, only a limited volume can be injected; hence high-titer purified recombinant adenoviruses are required. For example, Aizarani N et al introduced human urokinase-like plasminogen activator in an adenoviral vector intravenously [48]. Wienert B et al injected into the tail veins of nine- to eleven-week-old male mice a dose of 1 x 109 infection units (IFU) adenovirus in 200 µl PBS to edit Pcsk9 gene [11]. Retroorbital plexus is another site [49]. It is relatively easier than tail vein, but virus migrates to multiple organs systemically due to tropism and also due to the route of administration. There are also other invasive methods of adenoviral delivery requiring surgery which depends primarily on the research goal and type of organ to be infected and the life span of the experiment.
It is relatively easier than any method of intravenous delivery and researchers often use this method to avoid technically challenging intravenous routes of administration. The disadvantage of this method is that large volumes of the virus are required to be injected to facilitate transduction of a particular organ or tissue because the virus migrates in the system globally to all parts.
In addition to intravenous and intraperitoneal delivery, the adenoviral vector can also be delivered to the cerebrospinal fluid via cisterna magna [50].
All procedures during the generation and production of adenoviral vectors must be performed in a biosafety level 2 (BSL2) laboratory following the NIH guidelines and should be approved by the institutional biosafety committee. The critical points of the biosafety requirements are the use of biosafety cabinet hoods, the establishment of proper procedures for decontamination of any pieces of equipment and tools and disinfection of contaminated surfaces. Besides, all solid and liquid waste generated during any process should be adequately decontaminated and subsequently disposed of. The institute requires an established, detail-oriented protocol for the use of adenoviral vectors both in vitro and in vivo addressing how the experiments will be performed including explicit details of the maintenance and disposal of materials upon experiment completion.
There are commercial and non-profit institutes that provide services to provide custom adenoviruses, for example, Vector BioLabs in Philadelphia [50, 51], and SignaGen Laboratories [51] in Rockville, Maryland. The drawback as opposed to generating the vector in a lab is that these services are costly, especially if a researcher performs multiple in vivo experiments. Table 3 lists some of the services.
| Commercial Services | Non-profit Services | |
|---|---|---|
| Uses | Premade or custom Adviruses, individual vectors and components for generating custom Adviruses in the lab | Premade or custom Adviruses, individual vectors and components for generating custom Adviruses in the lab |
| Pros | Fast, efficient service | Free or subsidized |
| Cons | Expensive, good for one time use in vivo | Time constraint, paperwork and clearance for authorization |
| Companies | Vector BioLabs [11, 51], SignaGen Laboratories [51], Virapur, Viraquest, Creative biogene, Seven Hills Bioreagents | Stanford Gene Vector and Virus Core [52], Upenn vector core [53], U Michigan vector core, BCM vector core, ATCC, U Iowa vector core [54] |
- Hilleman M, Werner J. Recovery of new agent from patients with acute respiratory illness. Proc Soc Exp Biol Med. 1954;85:183-8 pubmed
- Flewett T, Bryden A, Davies H. Letter: Virus particles in gastroenteritis. Lancet. 1973;2:1497 pubmed
- Hierholzer J. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5:262-74 pubmed
- Wadell G, Hammarskjold M, Winberg G, Varsanyi T, Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16-42 pubmed
- Russell W. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573-604 pubmed
- Wilson J. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185-7 pubmed
- Nabel G. Development of optimized vectors for gene therapy. Proc Natl Acad Sci U S A. 1999;96:324-6 pubmed
- Stewart P, Burnett R, Cyrklaff M, Fuller S. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145-54 pubmed
- Nemerow G. Cell receptors involved in adenovirus entry. Virology. 2000;274:1-4 pubmed
- Philipson L, Lonberg Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064-75 pubmed
- Chinnadurai G, Chinnadurai S, Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979;32:623-8 pubmed
- Carlock L, Jones N. Transformation-defective mutant of adenovirus type 5 containing a single altered E1a mRNA species. J Virol. 1981;40:657-64 pubmed
- Solnick D. An adenovirus mutant defective in splicing RNA from early region 1A. Nature. 1981;291:508-10 pubmed
- McGrory W, Bautista D, Graham F. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614-7 pubmed
- Ketner G, Spencer F, Tugendreich S, Connelly C, Hieter P. Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc Natl Acad Sci U S A. 1994;91:6186-90 pubmed
- Graham F, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3:207-20 pubmed
- Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805-10 pubmed
- Parks R, Chen L, Anton M, Sankar U, Rudnicki M, Graham F. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565-70 pubmed
- Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci U S A. 1996;93:1320-4 pubmed
- He T, Zhou S, da Costa L, Yu J, Kinzler K, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509-14 pubmed
- Zeng M, Smith S, Siegel F, Shi Z, Van Kampen K, Elmets C, et al. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260-2 pubmed
- Luo J, Deng Z, Luo X, Tang N, Song W, Chen J, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236-47 pubmed
- McConnell M, Imperiale M. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15:1022-33 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- genereagentmethod
- Adeno-Associated Viral-Mediated Gene Transfer
- Assay Development: 5 Considerations and 8 Fundamentals
- Cell Lines Companies
- Cloning and Expression Vectors, and cDNA and microRNA Clones Companies
- Nucleic Acid Delivery: Lentiviral and Retroviral Vectors
- Protein Expression
- siRNAs and shRNAs: Tools for Protein Knockdown by Gene Silencing
