A comprehensive review of phosphotyrosine antibodies including a summary of phosphotyrosine antibodies cited among the over 60,000 formal publications in Labome's Validated Antibody Database.
Tyrosine phosphorylation is one of the major means of cell signaling transduction and enzymatic activity regulation. The advent of anti-phosphotyrosine antibodies is a significant event in signal transduction research. Before the availability of anti-phosphotyrosine antibodies, tyrosyl phosphorylation of proteins and enzymes was investigated through hazardous and time-consuming radioactive experiments [1]. Figure 1 exemplifies such an experiment. Anti-phosphotyrosine antibodies are commonly used in western blots after the targeted protein has been immunoprecipitated to measure the tyrosyl phosphorylation of the protein. For example, van de Kooij B et al immunoprecipitated FLAG-Nek10 after an in vitro kinase reaction with an anti-FLAG antibody and detected the tyrosine phosphorylation of Ned10 with Cell Signaling Technology rabbit monoclonal anti-phosphotyrosine antibody ( 8954) at 1:2000 dilution [3]. Anti-phosphotyrosine antibodies are also used on cell lysates to examine the overall change of tyrosine phosphorylation level in response to treatments.

Reviews of other non-gene-specific antibodies are available: tag antibodies ( HA, c-Myc and His6), western blot loading controls ( beta-actin, alpha tubulin), and secondary antibodies.
Three clones are commonly used: 4G10, PY20, and p-TYR-100. PY20 clone of IgG2b isotype was first developed and described by Dr. John R. Glenney, Jr and his colleagues in 1988 [4]. The other two clones were developed by commercial identities (MilliporeSigma/Upstate 4G10 and New England Biolabs/Cell Signaling Technology p-TYR-100).
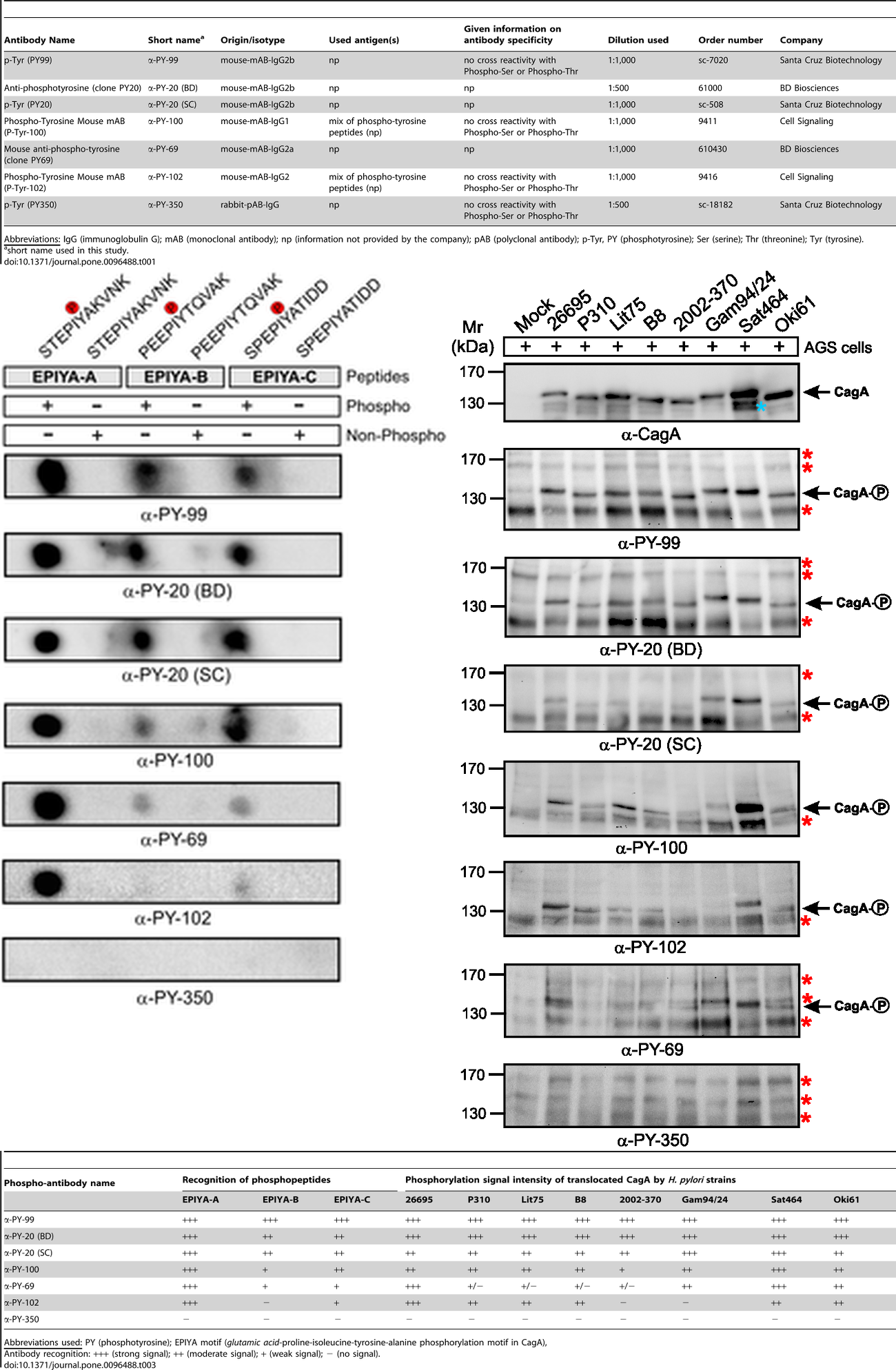
Anti-phosphotyrosine antibodies binding to tyrosine-phosphorylated peptides exhibit substantial sequence selectivity. Michele Tinti et al demonstrated that different phosphotyrosine antibody clones recognized their target sites in a sequence-specific manner and that this recognition differed from each other [5]. According to peptide microarray data, although different clones (e.g. 4G10, pY20 and p-TYR-100) showed a common enrichment of prolines at position +3 and leucine at position -1 concerning the phosphorylated tyrosine in the peptides, differences between the sequence preferences were observed. For example, clone 4G10 displayed a sequence context preference for Pro, Thr, Val and Phe at position -3. Both 4G10 and p-TYR-100 bindings were likely to be hindered by a negative charge at position -1, while pY20 was more sensitive to a positive charge at the same position. Among phosphotyrosine peptides, a total of 10%-20% could be uniquely recognized by one antibody. These specificity differences were reflected in Western blots, where band intensities of 4G10 and p-TYR-100 differed and pY20 even stained an additional group of 95–34 kDa proteins [5]. This substantial sequence selectivity is further evidenced by a direct comparison of seven commercial antibodies with regards to synthetic phospho-peptides in dot blots and phosphorylated CagA protein in Western blots (Figure 2) [2]. Performance of anti-phosphotyrosine antibodies has been known to vary widely between batches [6].
For mass spec phosphoproteomic analyses, a mixture of individual anti-phosphotyrosine monoclonals, termed Phospho-Tyrosine (P-Tyr-1000) MultiMab™ Rabbit mAb mix available from Cell Signaling Technology, has been shown to perform better than the commonly used 4G10 antibody clone [7]. The mixture enabled the identification of 689 phosphopeptides with 60% ID reproducibility while 4G10 clone achieved a total of 421 at 46% ID reproducibility [2]. Lundby A et al used such a mixture to pulldown phopho-tyrosine proteins from mouse lung lysates [8].
| Supplier | Num |
|---|---|
| Cell Signaling Technology | 52 |
| Santa Cruz Biotechnology | 47 |
| BD Biosciences | 17 |
| Invitrogen | 8 |
| Abcam | 7 |
| BioLegend | 1 |
Labome surveys formal publications using phosphotyrosine antibodies in western blot, immunoprecipitation, immunocytochemistry, and other immunological technologies, to facilitate the identification of the best-suited phosphotyrosine antibodies. Phosphotyrosine antibodies can be obtained from many suppliers. Table 1 lists the suppliers of phosphotyrosine antibodies from this survey. Most articles, among those that cite a catalog number or clone name, list 4G10, and a few list PY20 clone (one article listed both clones [9] ). Table 2 lists the major applications of phosphotyrosine antibodies among the publications since 2014. The following text discusses some of the applications in earlier publications.
MilliporeSigma (originally from Upstate Biotechnology) has provided some of most commonly used anti-phospho-tyrosine antibodies, including the most widely used clone 4G10. Antibodies with clone 4G10 have been generated from both hybridoma and recombinant technology.
| Method | Supplier | Catalog number | Sample reference |
|---|---|---|---|
| ELISA | Invitrogen | 03-7722 | [10, 11] |
| IC | Cell Signaling Technology | 9411 | [12, 13] |
| IC | Santa Cruz Biotechnology | sc-7020 | [14, 15] |
| IHC | Cell Signaling Technology | 9411 | [16, 17] |
| IHC-P | Cell Signaling Technology | 9415 | [17, 18] |
| IP | Cell Signaling Technology | 9411 | [19, 20] |
| IP | Santa Cruz Biotechnology | sc-508 | [21, 22] |
| WB | Santa Cruz Biotechnology | sc-7020 | [23, 24] |
| WB | Cell Signaling Technology | 9411 | [25, 26] |
| WB | BD Biosciences | 610000 | [27, 28] |
| WB | Cell Signaling Technology | 8954 | [29, 30] |
| WB | Santa Cruz Biotechnology | sc-508 | [31, 32] |
| WB | Cell Signaling Technology | 9416 | [33, 34] |
| WB | Abcam | ab10321 | [35, 36] |
| WB | BD Biosciences | 610012 | [37, 38] |
| WB | Invitrogen | 03-7700 | [39, 40] |
| WB | Invitrogen | 03-7799 | [39, 40] |
| WB | Invitrogen | AHO0681 | [39, 40] |
| WB | Invitrogen | MA1-82787 | [39, 40] |
| WB | Santa Cruz Biotechnology | sc-7020 AC | [41, 42] |
B Kushawaha et al stained smeared slides with mouse monoclonal anti-phosphotyrosine antibody from MilliporeSigma (P3300, clone PT-66) [43]. Lundby A et al immunobloted the immunoprecipitates by CRK and CRKL antibodies from A549 cell lysates with MilliporeSigma 4G10 antibody ( 05-321) [8]. Dagliyan O et al blotted cell lysates and immunoprecipitated proteins with anti-pTyr (4G10) antibody from MilliporeSigma to measure the tyrosine kinase activity of Src variants [44]. Others used 4G10 Platinum as well [45].
Santa Cruz Biotechnology mouse monoclonal anti-phosphotyrosine antibody was used to perform western blot to investigate the phosphorylation of VEGFR-2 in RF/6A cells [46] and the roles of Vav2 and Vav3 in skin cancer [47].
Cell Signaling Technology phosphotyrosine antibody was used in western blot to study NeK10 [3] and in immunoprecipitation with PTMScan pTyr antibody beads (p-Tyr-1000) [45].
Some of the commonly asked questions are answered here.
There are several ways.
- use a phosphorylation-specific antibody against the specific protein.
- use an antibody against the specific protein to immunoprecipitate both phosphorylated or non-phosphorylated forms of the specific protein, then detect with phosphotyrosine antibodies in Western blot.
- use phosphotyrosine antibodies to immunoprecipitate all tyrosine-phosphorylated proteins, and then detect with an antibody against the specific protein.
- mass spec.
For a detailed discussion of the detection of protein phosphorylation, see Labome article about Protein Modification Research Methods.
BSA should be used since nonfat dry milk contains proteins that are phosphorylated at tyrosine residues.
The samples should generally be used fresh, since the proteins might be degraded, and dephosphorylation may occur due to phosphatases.
We do not know the answer to this question. Anti-phosphotyrosine antibodies have been used for Western blot, immunoprecipitation, immunocytochemistry, and immunohistochemistry.
- Ushiro H, Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980;255:8363-5 pubmed
- Blaivas J, Scott R, Labib K. Urodynamic evaluation as neurologic test of sacral cord function. Urology. 1979;13:682-7 pubmed
- Chen C, Chen N, Lau L. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443-52 pubmed
- Huang Y, Bulavin D. Oncogene-mediated regulation of p53 ISGylation and functions. Oncotarget. 2014;5:5808-18 pubmed
- Dagliyan O, Tarnawski M, Chu P, Shirvanyants D, Schlichting I, Dokholyan N, et al. Engineering extrinsic disorder to control protein activity in living cells. Science. 2016;354:1441-1444 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- method
- Antibody Applications
- Antibody Companies
- Antibody Dilution and Antibody Titer
- Antibody Quality
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- GFP Antibody
- HA Hemagglutinin Tag Antibody and FAQs
- Mouse Antibody
- Myc Antibody Review
- Rabbit Antibody
- Rat Antibody
- Recombinant Antibodies
- Secondary Antibodies Companies
- Secondary Antibodies
