A comprehensive review of the histidine tag and anti-polyhistidine antibodies including a summary of anti-polyhistidine antibodies cited among the over 60,000 formal publications in Labome's Validated Antibody Database.
Histidine tag (His, polyhistidine) is one of most commonly used protein/tags for protein expression and purification. The presence of his-tag can be easily examined through anti-His-tag antibodies.
Both polyclonal and monoclonal antibodies against the polyhistidine tag are available commercially. The small polyhistidine tag with six histidine residues is not immunogenic; therefore a carrier protein is conjugated to the tag to elicit an immune response in a host animal. Keyhole limpet hemocyanin protein (KLH) is a very commonly used carrier. KLH is a very large, multisubunit, heterogeneously glycosylated, oxygen-carrying protein found in the gastropod, giant keyhole limpet (Megathura crenulata). It is highly immunogenic and offers a large number of potential sites for the conjugation of small tags. As gastropods are phylogenetically distant from most commonly used model systems, the use of KLH as a carrier dramatically minimizes the probability of cross-reactivity and false positives.
Anti-polyhistidine antibodies (also called anti-His antibodies) are suitable for use on western blots, immunosorbent (ELISA) assays, immunofluorescence assays, and immunoprecipitations. Figure 1 shows a typical immunocytochemical experiment with an anti-polyhistidine antibody. The antibody can also be coupled to magnetic beads for immunoprecipitations or to horseradish peroxidase (HRP) for direct detection of histidine-tagged proteins on western and dot blots, ELISA, for immunohistochemistry and immunocytochemistry. Anti-polyhistidine antibodies that have been conjugated to certain dyes are also available for time-resolved fluorescence energy transfer (TR-FRET) assays (Figure 2). FRET is based on energy transfer from a donor to an acceptor molecule when they are present nearby. For FRET assays, anti-histidine antibodies can be conjugated to donor and acceptor dyes. Anti-polyhistidine antibody tagged to europium (Eu) chelate can be used as a donor molecule. Eu chelate dyes have an exceptionally long emission lifetime, which makes them ideally suited for even delayed FRET measurements. The anti-polyhistidine antibodies can also be conjugated to an acceptor dye, allophycocyanin (APC). APC is bulky and can potentially create a steric hindrance. As an alternative, PerkinElmer provides ULight, a small acceptor dye whose spectral properties are similar to APC. Some of the anti-polyhistidine antibodies are terminal-specific, only recognizing histidine tags when they are in the C- or N-terminal of the expressed proteins.

Since some endogenous proteins may also contain stretches of histidine residues [2], they can also be recognized by anti-polyhistidine antibodies. One such an example protein is the human transcription regulator YY1, which contains 11 histidine residues from amino acid 70 to 80 [3]. This possibility of endogenous, non-specific signals has implications, especially in the cases of immunocytochemistry and immunocytochemistry applications.
| Supplier | Num |
|---|---|
| Invitrogen | 71 |
| Santa Cruz Biotechnology | 15 |
| Abcam | 11 |
| Sino Biological | 5 |
| Abcepta | 4 |
| Bio-Rad | 4 |
| Cell Signaling Technology | 4 |
| GE Healthcare Life Biosciences | 4 |
| MBL International | 2 |
| OriGene | 1 |
Labome surveys formal publications that have used anti-polyhistidine antibodies from various suppliers. Table 1 lists the suppliers of anti-polyhistidine antibodies in Labome's Validated Antibody Database (VAD). Table 2 displays the most cited catalog numbers for specific methods. Both Table 1 and 2 are based on articles published since 2014. Discussed briefly below are the applications of anti-polyhistidine antibodies from earlier publications or recent individual articles.
| Method | Supplier | Catalog number | Sample reference |
|---|---|---|---|
| ELISA | Sino Biological | 105327-MM02T | [4, 5] |
| ELISA | Invitrogen | MA1-21315 | [6, 7] |
| ELISA | Sino Biological | 105327-MM02T-H | [8, 9] |
| ELISA | Bio-Rad | MCA1396 | [10, 11] |
| IC | Invitrogen | R930-25 | [12, 13] |
| IHC-P | Invitrogen | MA1-21315 | [14, 15] |
| IP | Invitrogen | MA1-21315 | [16, 17] |
| IP | Abcam | ab18184 | [18, 19] |
| IP | Bio-Rad | MCA1396 | [20, 21] |
| IP | Invitrogen | MA1-21315-1MG | [16, 22] |
| WB | Invitrogen | MA1-21315 | [23, 24] |
| WB | Santa Cruz Biotechnology | sc-8036 | [25, 26] |
| WB | Invitrogen | 37-2900 | [27, 28] |
| WB | Invitrogen | MA1-21315-1MG | [29, 30] |
| WB | GE Healthcare Life Biosciences | 27-4710-01 | [31, 32] |
| WB | Invitrogen | MA1-21315-HRP | [33, 34] |
| WB | Invitrogen | R931-25 | [35, 36] |
| WB | Invitrogen | R940-25 | [37, 38] |
| WB | Abcam | ab18184 | [39, 40] |
| WB | Abcepta | AM1010a | [41, 42] |
| WB | Invitrogen | R930-25 | [43, 44] |
| WB | Bio-Rad | MCA1396 | [10, 21] |
| WB | Cell Signaling Technology | 2366 | [45, 46] |
| WB | MBL International | D291-3 | [47, 48] |
Arboleda-Velasquez JF et al used Novus Biologicals rabbit monoclonal anti-his antibody NBP2-61482 for Western blot and ELISA [49].
Steichen JM et al screened scFv libraries labelled with HIS-tagged Env proteins using Miltenyi Biotech anti-HIS PE antibody [50].
New England Biolabs anti-histidine antibody was used to perform western blots to show that in E. coli, some mRNAs are capable of being transported to sites where their proteins are needed without being translated [51]. Its mouse monoclonal anti-His6X antibody was used to perform immunoprecipitation to investigate the role of GSK3-TIP60-ULK1 signaling pathway during autophagy [52]. Steichen JM et al used a rabbit anti-His polyclonal antibody from Genscript (A00174) to pre-coat ELISA plates to assess monoclonal antibody binding [50]. Cell Signaling Technology D3I1O rabbit clone is another popular choice [53].
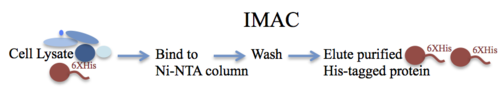
A detailed understanding of many biological processes requires expression and purification of recombinant proteins. Immobilized metal affinity chromatography (IMAC) is a powerful method that allows quick and efficient purification of recombinant proteins that have been tagged with a short affinity tag containing multiple histidine residues (Figure 3). The concept of IMAC was first developed and demonstrated by Porath et al. in 1975. Since then, IMAC has been widely used for the purification of a variety of His-tagged proteins and has also found important applications in immunosorbent assays and chip-based assays [54].
IMAC is based on the interactions between a metal ion that has been immobilized on a matrix and the histidine side chain. Histidine exhibits strong interaction with metal ions, such as Co2+, Ni2+, Cu2+, and Zn2+. The histidine imidazole ring readily forms coordination bonds with these metal ions, which are immobilized on a matrix with a chelating group (such as iminodiacetic acid (IDA) or nitrilotriacetic acid (NTA)) via a long hydrophilic spacer. The spacer ensures that the metal ions are easily accessible to the histidine tags. The histidine-tagged proteins are selectively and efficiently retained on the chromatography column matrix in the pH range of 7 to 8. The tagged proteins are easily eluted by lowering the pH, and optimal purification is achieved with a decreasing pH gradient. Imidazole competes with histidine for binding to metal ions; therefore, imidazole gradient is often used for eluting the protein of interest.
Expression systems, such as E. coli, S. cerevisiae, baculovirus infected insect cells, and mammalian cells have been used for the purification of polyhistidine-tagged proteins. Specialized E coli strains can be used for incorporating radio-labelled amino acids [55]. LOBSTR is an optimized E. coli strain for His-tag protein expression, especially those low-expressing proteins [56]. Polyhistidine tag, comprising of six histidine residues, is commonly used for affinity purification, and longer, as well as shorter tags, have also been used successfully. The polyhistidine tag may be placed at the N- or the C-terminal of the protein. The tag may also be placed internally at any appropriate location in the protein. For example, Winkler MBL et al expressed NCR1 and 2 genes in the yeast expression strain DSY-5 with a C-terminal thrombin cleavage site and deca-histidine tag [57]. It is important that the tag is easily accessible for interacting with the metal ions, and optimal placement of the polyhistidine tag depends on the protein of interest. As the polyhistidine tag is small, it usually does not interfere with protein function. However, there have been cases where the small His-tag affects protein structure and activity. For example, de Almeida JM et al. assessed the effect of an N-terminal His-tag on the structure, activity, stability and immobilization of LipC12, a highly active lipase, and found that the presence of the His-tag reduced its hydrolytic activities against natural and artificial substrates, among other effects [58]. Booth WT et al. evaluated the efffect of an N-terminal polyhistidine tag on the thermal stability of ten proteins through differential scanning fluorimetry and found the presence of His-tag mostly decreased protein thermal stability [59].
His-tag may need to be removed for certain applications, such as mass spectrometry or protein crystallization, with a protease, for example, His-3C protease [60]. A protease cleavage site can be built into the cloning vector that allows easy removal of the tag following protein purification. The polyhistidine-tagged proteins that remain soluble can usually be purified under native conditions [61]. However, proteins that aggregate or form inclusion bodies may be purified under denaturing conditions using urea or guanidinium hydrochloride without affecting the affinity of the tag for the metal ions [61]. Specialized columns, such as GE Healthcare HisTrap HP or HiTrap Q HP [62] or resins like Roche cOmplete His-Tag Purification Resin [63], can be used to purify expressed proteins easily. On the other hand, His-tag can stay on the expressed protein with any downstream effect. For example, the editing efficiencies of prime editing protein with and without an His-tag in human cells were similar [64].
IMAC offers a robust and versatile method for purification of proteins. It is one of the most widely used chromatography methods. The polyhistidine tag is easily added to proteins of interest; it rarely interferes with protein function and provides high purity and excellent recovery in a single purification step. The elution condition is very mild; then the His-tag can be used to prepare active proteins. Imidazole used to elute His-tagged proteins can be removed from proteins by dialysis or buffer exchange. A significant disadvantage is the non-specific binding of other proteins to the matrix and subsequent co-elution. This is usually an issue with mammalian proteins, which tend to contain consecutive histidine residues. Non-specific hydrophobic interactions can also result in co-purification of contaminating proteins. The inclusion of non-ionic detergents, sodium chloride, glycerol or ethanol in the buffers can minimize non-specific interactions [61]. The polyhistidine tag can be easily combined with other tags on the same protein. The multi-tag system allows purification under a variety of conditions to improve purity and yield.
Yes, polyhistidine-tagged membrane proteins have been successfully purified using buffers that contain ionic and nonionic detergents.
The formation of disulfide bonds with the protein of interest may lead to co-elution. Including 2-mercaptoethanol in the buffers usually resolves this problem. Non-specific interactions will also lead to the co-elution of contaminating proteins. The inclusion of a nonionic detergent can significantly reduce the binding of non-specific proteins.
Most anti-histidine antibodies recognize N- and C-terminal tagged polyhistidine tags as well as those present internally. Please refer to the supplier’s website for detailed information. Most suppliers also list which applications their antibodies are most suited for. Many suppliers also provide customer support services, which will answer queries regarding specific applications, antibody concentration, and troubleshooting.
Multiple bands are usually a result of non-specific binding. Reducing the concentration of the anti-histidine antibody or the secondary antibody or both often helps in reducing non-specific binding. Including a small amount of detergent, such as Tween-20 to the primary and secondary antibody solutions can also help to eliminate the problem of multiple bands on a western blot. Tween-20 may also be included in the wash buffer, and increasing the number of washes can also help.
Cross-reactivity is an important consideration when working with antibodies, but usually, it can be successfully addressed by changing experimental conditions. However, some mammalian proteins contain two or more consecutive histidine residues, which may be recognized by anti-histidine antibodies leading to cross-reactivity.
- Andresen C, Smedegaard S, Sylvestersen K, Svensson C, Iglesias Gato D, Cazzamali G, et al. Protein interaction screening for the ankyrin repeats and suppressor of cytokine signaling (SOCS) box (ASB) family identify Asb11 as a novel endoplasmic reticulum resident ubiquitin ligase. J Biol Chem. 2014;289:2043-54 pubmed publisher
- Huang S, Zhou A, Nguyen D, Zhang H, Benz E. Protein 4.1R Influences Myogenin Protein Stability and Skeletal Muscle Differentiation. J Biol Chem. 2016;291:25591-25607 pubmed
- Lei S, Ramesh A, Twitchell E, Wen K, Bui T, Weiss M, et al. High Protective Efficacy of Probiotics and Rice Bran against Human Norovirus Infection and Diarrhea in Gnotobiotic Pigs. Front Microbiol. 2016;7:1699 pubmed
- Horvath M, Mihajlovic Z, Slaninova V, Perez Gomez R, Moshkin Y, Krejci A. The silent information regulator 1 (Sirt1) is a positive regulator of the Notch pathway in Drosophila. Biochem J. 2016;473:4129-4143 pubmed
- Poornima G, Shah S, Vignesh V, Parker R, Rajyaguru P. Arginine methylation promotes translation repression activity of eIF4G-binding protein, Scd6. Nucleic Acids Res. 2016;44:9358-9368 pubmed
- Bornhorst J, Falke J. Purification of proteins using polyhistidine affinity tags. Methods Enzymol. 2000;326:245-54 pubmed
- Materials and Methods [ISSN : 2329-5139] is a unique online journal with regularly updated review articles on laboratory materials and methods. If you are interested in contributing a manuscript or suggesting a topic, please leave us feedback.
- reagentmethod
- Anti-GPCR Antibodies
- Antibody Companies
- Antibody Conjugation
- Antibody Storage and Antibody Shelf Life
- Antibody Structure and Antibody Fragments
- Beta Actin Antibody
- GFP Antibody
- Mouse Antibody
- Myc Antibody Review
- Phosphotyrosine Antibody
- Protein/Peptide Tags
- Protein Purification
- Rabbit Antibody
- Rat Antibody
- Secondary Antibodies Companies
